Outcomes of adults with acute lymphoblastic leukemia relapsing after allogeneic hematopoietic stem cell transplantation
- PMID: 23644077
- PMCID: PMC8439154
- DOI: 10.1016/j.bbmt.2013.04.014
Outcomes of adults with acute lymphoblastic leukemia relapsing after allogeneic hematopoietic stem cell transplantation
Abstract
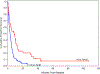
For patients with acute lymphoblastic leukemia (ALL) who relapse after allogeneic hematopoietic stem cell transplantation (HSCT), treatment options are limited, and the clinical course and prognostic factors affecting outcome have not been well characterized. We retrospectively analyzed outcomes of 123 adult patients with ALL who relapsed after a first HSCT performed at our center between 1993 and 2011. First-line salvage included second HSCT (n = 19), donor lymphocyte infusion with or without prior chemotherapy (n = 11), radiation therapy (n = 6), cytoreductive chemotherapy (n = 30), mild chemotherapy (n = 27), or palliative care (n = 23), with median postrelapse overall survival (OS) of 10 months, 6.5 months, 3 months, 4 months, 4 months, and 1 month, respectively. Despite a complete remission rate of 38% after first-line salvage in the treated patients, the OS rate remained limited with 1- and 2- year OS rates of 17% (95% confidence interval, 13 to 29) and 10% (95% confidence interval, 6 to 20), respectively. On univariate analysis, adverse factors for OS included active disease at the time of first HSCT and short time to progression from first HSCT (<6 months). There was no difference in the 6-month survival postrelapse in patients with isolated extramedullary relapse (44%) compared with combined extramedullary and bone marrow relapse (29%) or those with isolated bone marrow relapse (34%) (P = .8). Our data provide more insight into the disease behavior and treatment outcomes of ALL at relapse after HSCT against which future trials may be compared.
Copyright © 2013 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Haïat S, Marjanovic Z, Lapusan S, et al. Outcome of 40 adults aged from 18 to 55 years with acute lymphoblastic leukemia treated with double-delayed intensification pediatric protocol. Leukemia Research. 2011;35: 66–72. - PubMed
-
- DeAngelo DJ, Dahlberg S, Silverman LB, et al. A Multicenter Phase II Study Using a Dose Intensified Pediatric Regimen in Adults with Untreated Acute Lymphoblastic Leukemia. ASH Annual Meeting Abstracts. 2007;110: 587-.
-
- Bruggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107: 1116–1123. - PubMed
-
- Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. 2009;113: 4153–4162. - PubMed
-
- Kantarjian H, Thomas D, Jorgensen J, et al. Inotuzumab ozogamicin, an anti-CD22–calicheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. The Lancet Oncology. 2012;13: 403–411. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources