Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence
- PMID: 23644284
- PMCID: PMC3863361
- DOI: 10.1016/j.expneurol.2013.04.011
Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence
Abstract
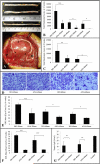
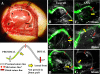
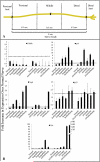
Repair of large nerve defects with acellular nerve allografts (ANAs) is an appealing alternative to autografting and allotransplantation. ANAs have been shown to be similar to autografts in supporting axonal regeneration across short gaps, but fail in larger defects due to a poorly-understood mechanism. ANAs depend on proliferating Schwann cells (SCs) from host tissue to support axonal regeneration. Populating longer ANAs places a greater proliferative demand on host SCs that may stress host SCs, resulting in senescence. In this study, we investigated axonal regeneration across increasing isograft and ANA lengths. We also evaluated the presence of senescent SCs within both graft types. A sciatic nerve graft model in rats was used to evaluate regeneration across increasing isograft (~autograft) and ANA lengths (20, 40, and 60 mm). Axonal regeneration and functional recovery decreased with increased graft length and the performance of the isograft was superior to ANAs at all lengths. Transgenic Thy1-GFP rats and qRT-PCR demonstrated that failure of the regenerating axonal front in ANAs was associated with increased levels of senescence related markers in the graft (senescence associated β-galactosidase, p16(INK4A), and IL6). Lastly, electron microscopy (EM) was used to qualitatively assess senescence-associated changes in chromatin of SCs in each graft type. EM demonstrated an increase in the presence of SCs with abnormal chromatin in isografts and ANAs of increasing graft length. These results are the first to suggest that SC senescence plays a role in limited axonal regeneration across nerve grafts of increasing gap lengths.
Keywords: 4′,6-diamidino-2-phenylindole; ANAs; Acellular nerve allograft; Cellular senescence; DAPI; EDL; GFP; Nerve autograft; Nerve grafting; Peripheral nerve; SCs; Schwann cell senescence; Schwann cells; SenScs; acellularized nerve allografts; extensor digitorum longus; green fluorescent protein; senescent Schwann cells.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


References
-
- Ansselin A, Pollard J. Immunopathological factors in peripheral nerve allograft rejection: quantification of lymphocyte invasion and major histocompatibility complex expression. J. Neurol. Sci. 1990;96:75–88. - PubMed
-
- Aydin MA, Mackinnon SE, Gu XM, Kobayashi J, Kuzon WM., Jr. Force deficits in skeletal muscle after delayed reinnervation. Plast. Reconstr. Surg. 2004;113:1712–1718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
