Expansion of oligodendrocyte progenitor cells following SIRT1 inactivation in the adult brain
- PMID: 23644469
- PMCID: PMC4026158
- DOI: 10.1038/ncb2735
Expansion of oligodendrocyte progenitor cells following SIRT1 inactivation in the adult brain
Abstract
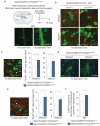
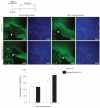
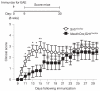
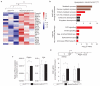
Oligodendrocytes-the myelin-forming cells of the central nervous system-can be regenerated during adulthood. In adults, new oligodendrocytes originate from oligodendrocyte progenitor cells (OPCs), but also from neural stem cells (NSCs). Although several factors supporting oligodendrocyte production have been characterized, the mechanisms underlying the generation of adult oligodendrocytes are largely unknown. Here we show that genetic inactivation of SIRT1, a protein deacetylase implicated in energy metabolism, increases the production of new OPCs in the adult mouse brain, in part by acting in NSCs. New OPCs produced following SIRT1 inactivation differentiate normally, generating fully myelinating oligodendrocytes. Remarkably, SIRT1 inactivation ameliorates remyelination and delays paralysis in mouse models of demyelinating injuries. SIRT1 inactivation leads to the upregulation of genes involved in cell metabolism and growth factor signalling, in particular PDGF receptor α (PDGFRα). Oligodendrocyte expansion following SIRT1 inactivation is mediated at least in part by AKT and p38 MAPK-signalling molecules downstream of PDGFRα. The identification of drug-targetable enzymes that regulate oligodendrocyte regeneration in adults could facilitate the development of therapies for demyelinating injuries and diseases, such as multiple sclerosis.
Figures



Comment in
-
The quest for myelin in the adult brain.Nat Cell Biol. 2013 Jun;15(6):572-5. doi: 10.1038/ncb2750. Epub 2013 May 5. Nat Cell Biol. 2013. PMID: 23644465
References
-
- McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J. Neurochem. 2008;107:1–19. - PubMed
-
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat. Rev. Neurosci. 2008;9:839–855. - PubMed
-
- Nait-Oumesmar B, et al. Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur. J. Neurosci. 1999;11:4357–4366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

