The effect of two golimumab doses on radiographic progression in ankylosing spondylitis: results through 4 years of the GO-RAISE trial
- PMID: 23644549
- PMCID: PMC4033110
- DOI: 10.1136/annrheumdis-2012-203075
The effect of two golimumab doses on radiographic progression in ankylosing spondylitis: results through 4 years of the GO-RAISE trial
Abstract
Objective: To evaluate radiographic progression in patients with ankylosing spondylitis (AS) receiving two different doses of the tumour necrosis factor antagonist golimumab.
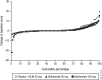
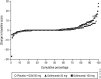
Methods: 356 patients with AS were randomly assigned to placebo, or golimumab 50 mg or 100 mg every 4 weeks (wks). At wk16, patients with inadequate response early escaped with blinded dose adjustments (placebo→golimumab 50 mg, 50 mg→100 mg). At wk24, patients still receiving placebo crossed over to golimumab 50 mg. Lateral view radiographs of the cervical/lumbar spine were obtained at wk0, wk104 and wk208, and scored (two blinded readers, modified Stoke AS Spine Score (mSASSS)). Observed data were used for wk104 analyses; missing wk208 scores were linearly extrapolated.
Results: Wk104 changes from baseline in mSASSS averaged 1.6±4.6 for placebo crossover, 0.9±2.7 for 50 mg and 0.9±3.9 for 100 mg. By wk208, following golimumab therapy for 3.5-4 years, mean changes in mSASSS were 2.1±5.2 for placebo crossover, 1.3±4.1 for 50 mg and 2.0±5.6 for 100 mg. Less than a third of patients (placebo crossover, 19/66 (28.8%); 50 mg, 29/111 (26.1%); 100 mg, 35/122 (28.7%)) had a definitive change from baseline mSASSS (>2). Less radiographic progression was observed through wk208 in patients without baseline syndesmophytes (0.2 vs 2.8 in patients with ≥1 syndesmophyte; p<0.0001) and with baseline C-reactive protein (CRP) levels ≤1.5 mg/dl (0.9 vs 2.9 with CRP >1.5 mg/dl; p=0.0004).
Conclusions: No difference in mSASSS change was observed between golimumab 50 mg and 100 mg. The radiographic progression rate remained stable at years 2 and 4, suggesting no acceleration of new bone formation over time. Golimumab-treated AS patients with no syndesmophytes and less systemic inflammation at baseline had considerably less radiographic progression.
Keywords: Ankylosing Spondylitis; Anti-TNF; Spondyloarthritis; TNF-alpha.
Figures

References
-
- Landewé R, Dougados M, Mielants H, et al. Physical function in ankylosing spondylitis is independently determined by both disease activity and radiographic damage of the spine. Ann Rheum Dis 2009;68:863–7 - PubMed
-
- van der Heijde D, Salonen D, Weissman BN, et al. ; for the Canadian (M03-606) study group and the ATLAS study group. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther 2009;11:R127. - PMC - PubMed
-
- van der Heijde D, Landewé R, Einstein S, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum 2008;58:1324–31 - PubMed
-
- van der Heijde D, Landewé R, Baraliakos X, et al. ; the Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy Study Group. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum 2008;58:3063–70 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

