Studies of IscR reveal a unique mechanism for metal-dependent regulation of DNA binding specificity
- PMID: 23644595
- PMCID: PMC3676455
- DOI: 10.1038/nsmb.2568
Studies of IscR reveal a unique mechanism for metal-dependent regulation of DNA binding specificity
Abstract
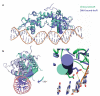
IscR from Escherichia coli is an unusual metalloregulator in that both apo and iron sulfur (Fe-S)-IscR regulate transcription and exhibit different DNA binding specificities. Here, we report structural and biochemical studies of IscR suggesting that remodeling of the protein-DNA interface upon Fe-S ligation broadens the DNA binding specificity of IscR from binding the type 2 motif only to both type 1 and type 2 motifs. Analysis of an apo-IscR variant with relaxed target-site discrimination identified a key residue in wild-type apo-IscR that, we propose, makes unfavorable interactions with a type 1 motif. Upon Fe-S binding, these interactions are apparently removed, thereby allowing holo-IscR to bind both type 1 and type 2 motifs. These data suggest a unique mechanism of ligand-mediated DNA site recognition, whereby metallocluster ligation relocates a protein-specificity determinant to expand DNA target-site selection, allowing a broader transcriptomic response by holo-IscR.
Figures
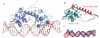

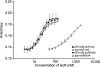


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

