Antioxidant systems from Pepper (Capsicum annuum L.): involvement in the response to temperature changes in ripe fruits
- PMID: 23644886
- PMCID: PMC3676799
- DOI: 10.3390/ijms14059556
Antioxidant systems from Pepper (Capsicum annuum L.): involvement in the response to temperature changes in ripe fruits
Abstract
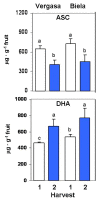
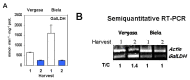
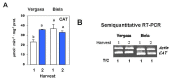
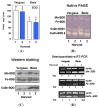
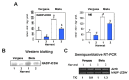
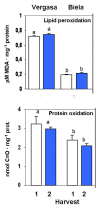
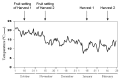
Sweet pepper is susceptible to changes in the environmental conditions, especially temperatures below 15 °C. In this work, two sets of pepper fruits (Capsicum annuum L.) which underwent distinct temperature profiles in planta were investigated. Accordingly, two harvesting times corresponding to each set were established: Harvest 1, whose fruits developed and ripened at 14.9 °C as average temperature; and Harvest 2, with average temperature of 12.4 °C. The oxidative metabolism was analyzed in all fruits. Although total ascorbate content did not vary between Harvests, a shift from the reduced to the oxidized form (dehydroascorbate), accompanied by a higher ascorbate peroxidase activity, was observed in Harvest 2 with respect to Harvest 1. Moreover, a decrease of the ascorbate-generating enzymatic system, the γ-galactono-lactone dehydrogenase, was found at Harvest 2. The activity values of the NADP-dependent dehydrogenases analyzed seem to indicate that a lower NADPH synthesis may occur in fruits which underwent lower temperature conditions. In spite of the important changes observed in the oxidative metabolism in fruits subjected to lower temperature, no oxidative stress appears to occur, as indicated by the lipid peroxidation and protein oxidation profiles. Thus, the antioxidative systems of pepper fruits seem to be involved in the response against temperature changes.
Figures



References
-
- Howard L.R., Talcott S.T., Brenes C.H., Villalon B. Changes in phytochemical and antioxidant activity of selected pepper cultivars (Capsicum species) as influenced by maturity. J. Agric. Food Chem. 2000;48:1713–1720. - PubMed
-
- Mateos R.M. Ph.D. Thesis. University of Granada; Spain: Jun 20, 2006. Pepper Antioxidants: Biochemical and Molecular Study of the Fruit Ripening and the Response to Abiotic Stress.
-
- Palma J.M., Corpas F.J., del Río L.A. Plant Vitamin Antioxidants and Their Influence on the Human Diet. In: Papareschi A., Eppolito H., editors. Fruit and Vegetable Consumption and Health. Nova Science Publishers, Inc; New York, NY, USA: 2009. pp. 127–138.
-
- Palma J.M., Jiménez A., Corpas F.J., Mateos R.M., Martí M.C., Sevilla F., del Río L.A. Role of ascorbate on the fruit physiology of pepper (Capsicum annuum L.) Funct. Plant Sci. Biotech. 2011;5:56–61.
-
- Martí M.C., Camejo D., Vallejo F., Romojaro F., Bacarizo S., Palma J.M., Sevilla F., Jiménez A. Influence of fruit ripening stage and harvest period on the antioxidant content of sweet pepper cultivars. Plant Foods Hum. Nutr. 2011;66:416–423. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

