Where do they come from and where do they go: candidates for regulating extracellular vesicle formation in fungi
- PMID: 23644887
- PMCID: PMC3676800
- DOI: 10.3390/ijms14059581
Where do they come from and where do they go: candidates for regulating extracellular vesicle formation in fungi
Abstract
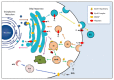
In the past few years, extracellular vesicles (EVs) from at least eight fungal species were characterized. EV proteome in four fungal species indicated putative biogenesis pathways and suggested interesting similarities with mammalian exosomes. Moreover, as observed for mammalian exosomes, fungal EVs were demonstrated to be immunologically active. Here we review the seminal and most recent findings related to the production of EVs by fungi. Based on the current literature about secretion of fungal molecules and biogenesis of EVs in eukaryotes, we focus our discussion on a list of cellular proteins with the potential to regulate vesicle biogenesis in the fungi.
Figures
References
-
- Latge J.P., Mouyna I., Tekaia F., Beauvais A., Debeaupuis J.P., Nierman W. Specific molecular features in the organization and biosynthesis of the cell wall of Aspergillus fumigatus. Med. Mycol. 2005;43:S15–S22. - PubMed
-
- Nimrichter L., Rodrigues M.L., Rodrigues E.G., Travassos L.R. The multitude of targets for the immune system and drug therapy in the fungal cell wall. Microbes Infect. 2005;7:789–798. - PubMed
-
- Barbosa M.S., Bao S.N., Andreotti P.F., de Faria F.P., Felipe M.S., dos Santos Feitosa L., Mendes-Giannini M.J., Soares C.M. Glyceraldehyde-3-phosphate dehydrogenase of Paracoccidioides brasiliensis is a cell surface protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect. Immun. 2006;74:382–289. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

