A facile synthesis of functionalized dispirooxindole derivatives via a three-component 1,3-dipolar cycloaddition reaction
- PMID: 23644979
- PMCID: PMC6270352
- DOI: 10.3390/molecules18055142
A facile synthesis of functionalized dispirooxindole derivatives via a three-component 1,3-dipolar cycloaddition reaction
Abstract
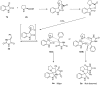
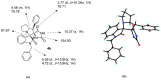
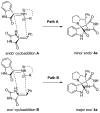
An efficient synthesis of novel dispirooxindoles has been achieved through three-component 1,3-dipolar cycloaddition of azomethine ylides generated in situ by the decarboxylative condensation of isatin and an α-amino acid with the dipolarophile 5-benzylideneimidazolidine-2,4-dione. The improved procedure features mild reaction conditions, high yields, high diastereoselectivities, a one-pot procedure and operational simplicity.
Figures
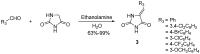
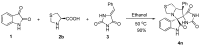
References
-
- Slobbe P., Ruijter E., Orru R.V.A. Recent applications of multicomponent reactions in medicinal chemistry. Medchemcomm. 2012;3:1189–1218.
-
- Burrell A.J.M., Coldham I., Watson L., Oram N., Pilgram C.D., Martin N.G. Stereoselective formation of fused tricyclic amines from acyclic aldehydes by a cascade process involving condensation, cyclization, and dipolar cycloaddition. J. Org. Chem. 2009;74:2290–2300. doi: 10.1021/jo8019913. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

