Magnetic resonance spectroscopy as an early indicator of response to anti-angiogenic therapy in patients with recurrent glioblastoma: RTOG 0625/ACRIN 6677
- PMID: 23645534
- PMCID: PMC3688017
- DOI: 10.1093/neuonc/not044
Magnetic resonance spectroscopy as an early indicator of response to anti-angiogenic therapy in patients with recurrent glioblastoma: RTOG 0625/ACRIN 6677
Abstract
Background: The prognosis for patients with recurrent glioblastoma remains poor. The purpose of this study was to assess the potential role of MR spectroscopy as an early indicator of response to anti-angiogenic therapy.
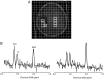
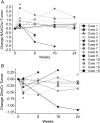
Methods: Thirteen patients with recurrent glioblastoma were enrolled in RTOG 0625/ACRIN 6677, a prospective multicenter trial in which bevacizumab was used in combination with either temozolomide or irinotecan. Patients were scanned prior to treatment and at specific timepoints during the treatment regimen. Postcontrast T1-weighted MRI was used to assess 6-month progression-free survival. Spectra from the enhancing tumor and peritumoral regions were defined on the postcontrast T1-weighted images. Changes in the concentration ratios of n-acetylaspartate/creatine (NAA/Cr), choline-containing compounds (Cho)/Cr, and NAA/Cho were quantified in comparison with pretreatment values.
Results: NAA/Cho levels increased and Cho/Cr levels decreased within enhancing tumor at 2 weeks relative to pretreatment levels (P = .048 and P = .016, respectively), suggesting a possible antitumor effect of bevacizumab with cytotoxic chemotherapy. Nine of the 13 patients were alive and progression free at 6 months. Analysis of receiver operating characteristic curves for NAA/Cho changes in tumor at 8 weeks revealed higher levels in patients progression free at 6 months (area under the curve = 0.85), suggesting that NAA/Cho is associated with treatment response. Similar results were observed for receiver operating characteristic curve analyses against 1-year survival. In addition, decreased Cho/Cr and increased NAA/Cr and NAA/Cho in tumor periphery at 16 weeks posttreatment were associated with both 6-month progression-free survival and 1-year survival.
Conclusion: Changes in NAA and Cho by MR spectroscopy may potentially be useful as imaging biomarkers in assessing response to anti-angiogenic treatment.
Keywords: Cho; NAA; anti-angiogenic therapy; bevacizumab; glioblastoma; magnetic resonance spectroscopy.
Figures

Similar articles
-
Early post-bevacizumab progression on contrast-enhanced MRI as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 Central Reader Study.Neuro Oncol. 2013 Jul;15(7):945-54. doi: 10.1093/neuonc/not049. Epub 2013 Jun 19. Neuro Oncol. 2013. PMID: 23788270 Free PMC article. Clinical Trial.
-
3-Dimensional magnetic resonance spectroscopic imaging at 3 Tesla for early response assessment of glioblastoma patients during external beam radiation therapy.Int J Radiat Oncol Biol Phys. 2014 Sep 1;90(1):181-9. doi: 10.1016/j.ijrobp.2014.05.014. Epub 2014 Jun 28. Int J Radiat Oncol Biol Phys. 2014. PMID: 24986746 Free PMC article.
-
Dynamic susceptibility contrast MRI measures of relative cerebral blood volume as a prognostic marker for overall survival in recurrent glioblastoma: results from the ACRIN 6677/RTOG 0625 multicenter trial.Neuro Oncol. 2015 Aug;17(8):1148-56. doi: 10.1093/neuonc/nou364. Epub 2015 Feb 2. Neuro Oncol. 2015. PMID: 25646027 Free PMC article. Clinical Trial.
-
[Drug therapy of patients with recurrent glioblastoma: is there any evidence?].Wien Med Wochenschr. 2011 Jan;161(1-2):26-31. doi: 10.1007/s10354-010-0863-5. Epub 2010 Dec 27. Wien Med Wochenschr. 2011. PMID: 21181283 Review. German.
-
Value of DWI Combined with Magnetic Resonance Spectroscopy in the Differential Diagnosis between Recurrent Glioma and Radiation Injury: A Meta-Analysis.Int J Clin Pract. 2022 Oct 25;2022:1629570. doi: 10.1155/2022/1629570. eCollection 2022. Int J Clin Pract. 2022. PMID: 36380750 Free PMC article. Review.
Cited by
-
Magnetic resonance spectroscopy outperforms perfusion in distinguishing between pseudoprogression and disease progression in patients with glioblastoma.Neurooncol Adv. 2022 Aug 15;4(1):vdac128. doi: 10.1093/noajnl/vdac128. eCollection 2022 Jan-Dec. Neurooncol Adv. 2022. PMID: 36071927 Free PMC article.
-
Individualized radiotherapy by combining high-end irradiation and magnetic resonance imaging.Strahlenther Onkol. 2016 Apr;192(4):209-15. doi: 10.1007/s00066-016-0944-5. Epub 2016 Feb 6. Strahlenther Onkol. 2016. PMID: 26852244 Review.
-
High-grade glioma management and response assessment-recent advances and current challenges.Curr Oncol. 2016 Aug;23(4):e383-91. doi: 10.3747/co.23.3082. Epub 2016 Aug 12. Curr Oncol. 2016. PMID: 27536188 Free PMC article. Review.
-
MR spectroscopic imaging predicts early response to anti-angiogenic therapy in recurrent glioblastoma.Neurooncol Adv. 2021 Apr 15;3(1):vdab060. doi: 10.1093/noajnl/vdab060. eCollection 2021 Jan-Dec. Neurooncol Adv. 2021. PMID: 34131648 Free PMC article.
-
Current Clinical Brain Tumor Imaging.Neurosurgery. 2017 Sep 1;81(3):397-415. doi: 10.1093/neuros/nyx103. Neurosurgery. 2017. PMID: 28486641 Free PMC article. Review.
References
-
- Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–2578. - PubMed
-
- Thaker NG, McDonald PR, Zhang F, et al. Designing, optimizing, and implementing high-throughput siRNA genomic screening with glioma cells for the discovery of survival genes and novel drug targets. J Neurosci Methods. 2010;185(2):204–212. - PubMed
-
- Cohen MH, Shen YL, Keegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14(11):1131–1138. - PubMed
-
- Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

