Antagonistic basic helix-loop-helix/bZIP transcription factors form transcriptional modules that integrate light and reactive oxygen species signaling in Arabidopsis
- PMID: 23645630
- PMCID: PMC3694698
- DOI: 10.1105/tpc.112.104869
Antagonistic basic helix-loop-helix/bZIP transcription factors form transcriptional modules that integrate light and reactive oxygen species signaling in Arabidopsis
Abstract
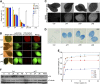
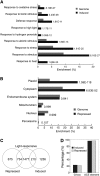
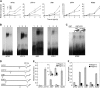
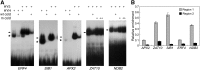
The critical developmental switch from heterotrophic to autotrophic growth of plants involves light signaling transduction and the production of reactive oxygen species (ROS). ROS function as signaling molecules that regulate multiple developmental processes, including cell death. However, the relationship between light and ROS signaling remains unclear. Here, we identify transcriptional modules composed of the basic helix-loop-helix and bZIP transcription factors PHYTOCHROME-INTERACTING FACTOR1 (PIF1), PIF3, ELONGATED HYPOCOTYL5 (HY5), and HY5 HOMOLOGY (HYH) that bridge light and ROS signaling to regulate cell death and photooxidative response. We show that pif mutants release more singlet oxygen and exhibit more extensive cell death than the wild type during Arabidopsis thaliana deetiolation. Genome-wide expression profiling indicates that PIF1 represses numerous ROS and stress-related genes. Molecular and biochemical analyses reveal that PIF1/PIF3 and HY5/HYH physically interact and coordinately regulate the expression of five ROS-responsive genes by directly binding to their promoters. Furthermore, PIF1/PIF3 and HY5/HYH function antagonistically during the seedling greening process. In addition, phytochromes, cryptochromes, and CONSTITUTIVE PHOTOMORPHOGENIC1 act upstream to regulate ROS signaling. Together, this study reveals that the PIF1/PIF3-HY5/HYH transcriptional modules mediate crosstalk between light and ROS signaling and sheds light on a new mechanism by which plants adapt to the light environments.
Figures



Comment in
-
Frenemies: antagonistic bHLH/bZIP transcription factors integrate light and reactive oxygen species signaling in Arabidopsis.Plant Cell. 2013 May;25(5):1483. doi: 10.1105/tpc.113.250510. Epub 2013 May 7. Plant Cell. 2013. PMID: 23653470 Free PMC article. No abstract available.
Similar articles
-
UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH.Plant Physiol. 2008 Feb;146(2):576-88. doi: 10.1104/pp.107.108456. Epub 2007 Nov 30. Plant Physiol. 2008. PMID: 18055587 Free PMC article.
-
Molecular interactions of GBF1 with HY5 and HYH proteins during light-mediated seedling development in Arabidopsis thaliana.J Biol Chem. 2012 Jul 27;287(31):25995-6009. doi: 10.1074/jbc.M111.333906. Epub 2012 Jun 12. J Biol Chem. 2012. PMID: 22692212 Free PMC article.
-
UV-B-responsive association of the Arabidopsis bZIP transcription factor ELONGATED HYPOCOTYL5 with target genes, including its own promoter.Plant Cell. 2014 Oct;26(10):4200-13. doi: 10.1105/tpc.114.130716. Epub 2014 Oct 28. Plant Cell. 2014. PMID: 25351492 Free PMC article.
-
Plant hormone signaling lightens up: integrators of light and hormones.Curr Opin Plant Biol. 2010 Oct;13(5):571-7. doi: 10.1016/j.pbi.2010.07.001. Epub 2010 Aug 23. Curr Opin Plant Biol. 2010. PMID: 20739215 Review.
-
The Multifaceted Roles of HY5 in Plant Growth and Development.Mol Plant. 2016 Oct 10;9(10):1353-1365. doi: 10.1016/j.molp.2016.07.002. Epub 2016 Jul 17. Mol Plant. 2016. PMID: 27435853 Review.
Cited by
-
Synergistic and Antagonistic Action of Phytochrome (Phy) A and PhyB during Seedling De-Etiolation in Arabidopsis thaliana.Int J Mol Sci. 2015 May 28;16(6):12199-212. doi: 10.3390/ijms160612199. Int J Mol Sci. 2015. PMID: 26030677 Free PMC article.
-
BrLETM2 Protein Modulates Anthocyanin Accumulation by Promoting ROS Production in Turnip (Brassica rapa subsp. rapa).Int J Mol Sci. 2021 Mar 29;22(7):3538. doi: 10.3390/ijms22073538. Int J Mol Sci. 2021. PMID: 33805479 Free PMC article.
-
RSM1, an Arabidopsis MYB protein, interacts with HY5/HYH to modulate seed germination and seedling development in response to abscisic acid and salinity.PLoS Genet. 2018 Dec 19;14(12):e1007839. doi: 10.1371/journal.pgen.1007839. eCollection 2018 Dec. PLoS Genet. 2018. PMID: 30566447 Free PMC article.
-
COP1 dynamics integrate conflicting seasonal light and thermal cues in the control of Arabidopsis elongation.Sci Adv. 2022 Aug 19;8(33):eabp8412. doi: 10.1126/sciadv.abp8412. Epub 2022 Aug 19. Sci Adv. 2022. PMID: 35984876 Free PMC article.
-
The miR396-GRFs Module Mediates the Prevention of Photo-oxidative Damage by Brassinosteroids during Seedling De-Etiolation in Arabidopsis.Plant Cell. 2020 Aug;32(8):2525-2542. doi: 10.1105/tpc.20.00057. Epub 2020 Jun 2. Plant Cell. 2020. PMID: 32487564 Free PMC article.
References
-
- Achard P., Renou J.-P., Berthomé R., Harberd N.P., Genschik P. (2008). Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 18: 656–660 - PubMed
-
- Alabadí D., Blázquez M.A. (2009). Molecular interactions between light and hormone signaling to control plant growth. Plant Mol. Biol. 69: 409–417 - PubMed
-
- Al-Sady B., Ni W., Kircher S., Schäfer E., Quail P.H. (2006). Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 23: 439–446 - PubMed
-
- Apel K., Hirt H.R. (2004). Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55: 373–399 - PubMed
-
- Bauer D., Viczián A., Kircher S., Nobis T., Nitschke R., Kunkel T., Panigrahi K.C., Adám E., Fejes E., Schäfer E., Nagy F. (2004). Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16: 1433–1445 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

