The bacterial translocon SecYEG opens upon ribosome binding
- PMID: 23645666
- PMCID: PMC3689939
- DOI: 10.1074/jbc.M113.477893
The bacterial translocon SecYEG opens upon ribosome binding
Abstract
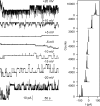
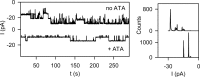
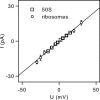
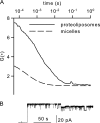
In co-translational translocation, the ribosome funnel and the channel of the protein translocation complex SecYEG are aligned. For the nascent chain to enter the channel immediately after synthesis, a yet unidentified signal triggers displacement of the SecYEG sealing plug from the pore. Here, we show that ribosome binding to the resting SecYEG channel triggers this conformational transition. The purified and reconstituted SecYEG channel opens to form a large ion-conducting channel, which has the conductivity of the plug deletion mutant. The number of ion-conducting channels inserted into the planar bilayer per fusion event roughly equals the number of SecYEG channels counted by fluorescence correlation spectroscopy in a single proteoliposome. Thus, the open probability of the channel must be close to unity. To prevent the otherwise lethal proton leak, a closed post-translational conformation of the SecYEG complex bound to a ribosome must exist.
Keywords: Channel Gating; Membrane Bilayer; Membrane Biophysics; Membrane Reconstitution; Permeability; Protein Translocation.
Figures


Similar articles
-
Competitive binding of the SecA ATPase and ribosomes to the SecYEG translocon.J Biol Chem. 2012 Mar 9;287(11):7885-95. doi: 10.1074/jbc.M111.297911. Epub 2012 Jan 20. J Biol Chem. 2012. PMID: 22267723 Free PMC article.
-
Ion conductivity of the bacterial translocation channel SecYEG engaged in translocation.J Biol Chem. 2014 Aug 29;289(35):24611-6. doi: 10.1074/jbc.M114.588491. Epub 2014 Jul 11. J Biol Chem. 2014. PMID: 25016015 Free PMC article.
-
Investigating the SecY plug movement at the SecYEG translocation channel.EMBO J. 2005 Oct 5;24(19):3380-8. doi: 10.1038/sj.emboj.7600804. Epub 2005 Sep 8. EMBO J. 2005. PMID: 16148946 Free PMC article.
-
The protein-conducting channel SecYEG.Biochim Biophys Acta. 2004 Nov 11;1694(1-3):81-95. doi: 10.1016/j.bbamcr.2004.02.009. Biochim Biophys Acta. 2004. PMID: 15546659 Review.
-
Translocation of proteins through the Sec61 and SecYEG channels.Curr Opin Cell Biol. 2009 Aug;21(4):501-7. doi: 10.1016/j.ceb.2009.04.010. Epub 2009 May 18. Curr Opin Cell Biol. 2009. PMID: 19450960 Free PMC article. Review.
Cited by
-
Structure of the native Sec61 protein-conducting channel.Nat Commun. 2015 Sep 28;6:8403. doi: 10.1038/ncomms9403. Nat Commun. 2015. PMID: 26411746 Free PMC article.
-
AFM-Based Force Spectroscopy Guided by Recognition Imaging: A New Mode for Mapping and Studying Interaction Sites at Low Lateral Density.Methods Protoc. 2019 Jan 8;2(1):6. doi: 10.3390/mps2010006. Methods Protoc. 2019. PMID: 31164590 Free PMC article.
-
Dynamic interaction of the sec translocon with the chaperone PpiD.J Biol Chem. 2014 Aug 1;289(31):21706-15. doi: 10.1074/jbc.M114.577916. Epub 2014 Jun 20. J Biol Chem. 2014. PMID: 24951590 Free PMC article.
-
YidC and SecYEG form a heterotetrameric protein translocation channel.Sci Rep. 2017 Mar 7;7(1):101. doi: 10.1038/s41598-017-00109-8. Sci Rep. 2017. PMID: 28273911 Free PMC article.
-
Tuning membrane protein mobility by confinement into nanodomains.Nat Nanotechnol. 2017 Mar;12(3):260-266. doi: 10.1038/nnano.2016.236. Epub 2016 Nov 14. Nat Nanotechnol. 2017. PMID: 27842062 Free PMC article.
References
-
- Driessen A. J., Nouwen N. (2008) Protein translocation across the bacterial cytoplasmic membrane. Annu. Rev. Biochem. 77, 643–667 - PubMed
-
- Rapoport T. A. (2007) Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature 450, 663–669 - PubMed
-
- Saparov S. M., Erlandson K., Cannon K., Schaletzky J., Schulman S., Rapoport T. A., Pohl P. (2007) Determining the conductance of the SecY protein translocation channel for small molecules. Mol. Cell 26, 501–509 - PubMed
-
- Simon S. M., Blobel G. (1992) Signal peptides open protein-conducting channels in E. coli. Cell 69, 677–684 - PubMed
-
- van den Berg B., Clemons W. M., Jr., Collinson I., Modis Y., Hartmann E., Harrison S. C., Rapoport T. A. (2004) X-ray structure of a protein-conducting channel. Nature 427, 36–44 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

