Vascular Ehlers-Danlos syndrome mutations in type III collagen differently stall the triple helical folding
- PMID: 23645670
- PMCID: PMC3696688
- DOI: 10.1074/jbc.M113.462002
Vascular Ehlers-Danlos syndrome mutations in type III collagen differently stall the triple helical folding
Abstract
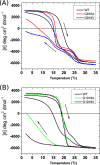
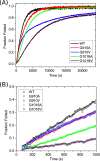
Vascular Ehlers-Danlos syndrome (EDS) type IV is the most severe form of EDS. In many cases the disease is caused by a point mutation of Gly in type III collagen. A slower folding of the collagen helix is a potential cause for over-modifications. However, little is known about the rate of folding of type III collagen in patients with EDS. To understand the molecular mechanism of the effect of mutations, a system was developed for bacterial production of homotrimeric model polypeptides. The C-terminal quarter, 252 residues, of the natural human type III collagen was attached to (GPP)7 with the type XIX collagen trimerization domain (NC2). The natural collagen domain forms a triple helical structure without 4-hydroxylation of proline at a low temperature. At 33 °C, the natural collagenous part is denatured, but the C-terminal (GPP)7-NC2 remains intact. Switching to a low temperature triggers the folding of the type III collagen domain in a zipper-like fashion that resembles the natural process. We used this system for the two known EDS mutations (Gly-to-Val) in the middle at Gly-910 and at the C terminus at Gly-1018. In addition, wild-type and Gly-to-Ala mutants were made. The mutations significantly slow down the overall rate of triple helix formation. The effect of the Gly-to-Val mutation is much more severe compared with Gly-to-Ala. This is the first report on the folding of collagen with EDS mutations, which demonstrates local delays in the triple helix propagation around the mutated residue.
Keywords: Collagen; Connective Tissue; Ehlers-Danlos Syndrome; Glycine Mutations; Mutant; Prolyl Isomerase; Protein Folding; Type III Collagen.
Figures





Similar articles
-
Impact of vascular Ehlers-Danlos Syndrome-associated Gly substitutions on structure, function, and mechanics using bacterial collagen.Matrix Biol. 2025 Feb;135:87-98. doi: 10.1016/j.matbio.2024.12.002. Epub 2024 Dec 6. Matrix Biol. 2025. PMID: 39645092
-
Haploinsufficiency for one COL3A1 allele of type III procollagen results in a phenotype similar to the vascular form of Ehlers-Danlos syndrome, Ehlers-Danlos syndrome type IV.Am J Hum Genet. 2001 Nov;69(5):989-1001. doi: 10.1086/324123. Epub 2001 Sep 27. Am J Hum Genet. 2001. PMID: 11577371 Free PMC article.
-
Stability related bias in residues replacing glycines within the collagen triple helix (Gly-Xaa-Yaa) in inherited connective tissue disorders.Hum Mutat. 2004 Oct;24(4):330-7. doi: 10.1002/humu.20091. Hum Mutat. 2004. PMID: 15365990
-
Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases.Gene. 2019 Jul 30;707:151-171. doi: 10.1016/j.gene.2019.05.003. Epub 2019 May 7. Gene. 2019. PMID: 31075413 Free PMC article. Review.
-
Vascular type of Ehlers-Danlos syndrome.J Nippon Med Sch. 2008 Oct;75(5):254-61. doi: 10.1272/jnms.75.254. J Nippon Med Sch. 2008. PMID: 19023163 Review.
Cited by
-
Whole-exome sequencing facilitates the differential diagnosis of Ehlers-Danlos syndrome (EDS).Mol Genet Genomic Med. 2022 Mar;10(3):e1885. doi: 10.1002/mgg3.1885. Epub 2022 Feb 4. Mol Genet Genomic Med. 2022. PMID: 35119775 Free PMC article.
-
Outcomes of vitrectomy for retinal detachment in a patient with Ehlers-Danlos syndrome type IV: a case report.J Med Case Rep. 2021 May 20;15(1):249. doi: 10.1186/s13256-021-02855-w. J Med Case Rep. 2021. PMID: 34011391 Free PMC article.
-
Inherited Disease Genetics Improves the Identification of Cancer-Associated Genes.PLoS Genet. 2016 Jun 15;12(6):e1006081. doi: 10.1371/journal.pgen.1006081. eCollection 2016 Jun. PLoS Genet. 2016. PMID: 27304678 Free PMC article.
-
The type of variants at the COL3A1 gene associates with the phenotype and severity of vascular Ehlers-Danlos syndrome.Eur J Hum Genet. 2015 Dec;23(12):1657-64. doi: 10.1038/ejhg.2015.32. Epub 2015 Mar 11. Eur J Hum Genet. 2015. PMID: 25758994 Free PMC article.
-
Decoding collagen's thermally induced unfolding and refolding pathways.Proc Natl Acad Sci U S A. 2025 May 20;122(20):e2420308122. doi: 10.1073/pnas.2420308122. Epub 2025 May 13. Proc Natl Acad Sci U S A. 2025. PMID: 40359051
References
-
- De Paepe A., Malfait F. (2012) The Ehlers-Danlos syndrome, a disorder with many faces. Clin. Genet. 82, 1–11 - PubMed
-
- Malfait F., De Paepe A. (2005) Molecular genetics in classic Ehlers-Danlos syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 139, 17–23 - PubMed
-
- Pinnell S. R., Krane S. M., Kenzora J. E., Glimcher M. J. (1972) A heritable disorder of connective tissue. Hydroxylysine-deficient collagen disease. N. Engl. J. Med. 286, 1013–1020 - PubMed
-
- Colige A., Nuytinck L., Hausser I., van Essen A. J., Thiry M., Herens C., Adès L. C., Malfait F., Paepe A. D., Franck P., Wolff G., Oosterwijk J. C., Smitt J. H., Lapière C. M., Nusgens B. V. (2004) Novel types of mutation responsible for the dermatosparactic type of Ehlers-Danlos syndrome (type VIIC) and common polymorphisms in the ADAMTS2 gene. J. Invest. Dermatol. 123, 656–663 - PubMed
-
- Baumann M., Giunta C., Krabichler B., Rüschendorf F., Zoppi N., Colombi M., Bittner R. E., Quijano-Roy S., Muntoni F., Cirak S., Schreiber G., Zou Y., Hu Y., Romero N. B., Carlier R. Y., Amberger A., Deutschmann A., Straub V., Rohrbach M., Steinmann B., Rostásy K., Karall D., Bönnemann C. G., Zschocke J., Fauth C. (2012) Mutations in FKBP14 cause a variant of Ehlers-Danlos syndrome with progressive kyphoscoliosis, myopathy, and hearing loss. Am. J. Hum. Genet. 90, 201–216 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

