Suppression of autophagy in osteocytes mimics skeletal aging
- PMID: 23645674
- PMCID: PMC3682543
- DOI: 10.1074/jbc.M112.444190
Suppression of autophagy in osteocytes mimics skeletal aging
Abstract
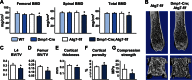
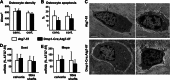
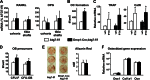
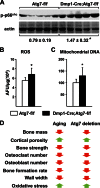
Bone mass declines with age but the mechanisms responsible remain unclear. Here we demonstrate that deletion of a conditional allele for Atg7, a gene essential for autophagy, from osteocytes caused low bone mass in 6-month-old male and female mice. Cancellous bone volume and cortical thickness were decreased, and cortical porosity increased, in conditional knock-out mice compared with control littermates. These changes were associated with low osteoclast number, osteoblast number, bone formation rate, and wall width in the cancellous bone of conditional knock-out mice. In addition, oxidative stress was higher in the bones of conditional knock-out mice as measured by reactive oxygen species levels in the bone marrow and by p66(shc) phosphorylation in L6 vertebra. Each of these changes has been previously demonstrated in the bones of old versus young adult mice. Thus, these results demonstrate that suppression of autophagy in osteocytes mimics, in many aspects, the impact of aging on the skeleton and suggest that a decline in autophagy with age may contribute to the low bone mass associated with aging.
Keywords: Aging; Autophagy; Bone; Mouse; Osteocyte; Remodeling.
Figures


References
-
- Manolagas S. C. (2000) Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 21, 115–137 - PubMed
-
- Riggs B. L., Melton L. J., Robb R. A., Camp J. J., Atkinson E. J., McDaniel L., Amin S., Rouleau P. A., Khosla S. (2008) A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J. Bone Miner. Res. 23, 205–214 - PMC - PubMed
-
- Almeida M., Han L., Martin-Millan M., Plotkin L. I., Stewart S. A., Roberson P. K., Kousteni S., O'Brien C. A., Bellido T., Parfitt A. M., Weinstein R. S., Jilka R. L., Manolagas S. C. (2007) Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 282, 27285–27297 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

