p39, the primary activator for cyclin-dependent kinase 5 (Cdk5) in oligodendroglia, is essential for oligodendroglia differentiation and myelin repair
- PMID: 23645679
- PMCID: PMC3689949
- DOI: 10.1074/jbc.M113.453688
p39, the primary activator for cyclin-dependent kinase 5 (Cdk5) in oligodendroglia, is essential for oligodendroglia differentiation and myelin repair
Abstract
Cyclin-dependent kinase 5 (Cdk5) plays key roles in normal brain development and function. Dysregulation of Cdk5 may cause neurodegeneration and cognitive impairment. Besides the well demonstrated role of Cdk5 in neurons, emerging evidence suggests the functional requirement of Cdk5 in oligodendroglia (OL) and CNS myelin development. However, whether neurons and OLs employ similar or distinct mechanisms to regulate Cdk5 activity remains elusive. We report here that in contrast to neurons that harbor high levels of two Cdk5 activators, p35 and p39, OLs express abundant p39 but negligible p35. In addition, p39 is selectively up-regulated in OLs during differentiation along with elevated Cdk5 activity, whereas p35 expression remains unaltered. Specific knockdown of p39 by siRNA significantly attenuates Cdk5 activity and OL differentiation without affecting p35. Finally, expression of p39, but not p35, is increased during myelin repair, and remyelination is impaired in p39(-/-) mice. Together, these results reveal that neurons and OLs harbor distinct preference of Cdk5 activators and demonstrate important functions of p39-dependent Cdk5 activation in OL differentiation during de novo myelin development and myelin repair.
Keywords: Cyclin-dependent Kinase 5 (Cdk5); Differentiation; Gene Regulation; Glia; Myelin; Neurons; Oligodendroglia; p39.
Figures
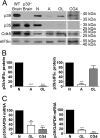
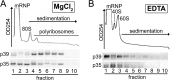

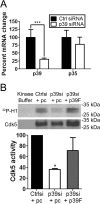

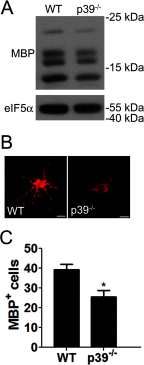

References
-
- Ren Y., Wang H., Xiao L. (2013) Improving myelin/oligodendrocyte-related dysfunction. A new mechanism of antipsychotics in the treatment of schizophrenia? Int. J. Neuropsychopharmacol. 16, 691–700 - PubMed
-
- Su S. C., Tsai L.-H. (2011) Cyclin-dependent kinases in brain development and disease. Annu. Rev. Cell Dev. Biol. 27, 465–491 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

