Brentuximab vedotin in relapsed/refractory Hodgkin's lymphoma: the Italian experience and results of its use in daily clinical practice outside clinical trials
- PMID: 23645687
- PMCID: PMC3729903
- DOI: 10.3324/haematol.2012.083048
Brentuximab vedotin in relapsed/refractory Hodgkin's lymphoma: the Italian experience and results of its use in daily clinical practice outside clinical trials
Abstract
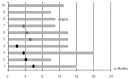
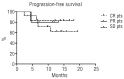
Clinical trial results indicate that brentuximab vedotin brings considerable promise for the treatment of patients with relapsed or refractory Hodgkin's lymphoma. A retrospective multicenter study was conducted on 65 heavily pretreated patients who underwent therapy through a Named Patient Program in Italy (non trial-setting). The primary study endpoint was the objective response rate; secondary endpoints were safety, overall survival and progression-free survival. The best overall response rate (70.7%), including 21.5% complete responses, was observed at the first restaging after the third cycle of treatment. After a median follow up of 13.2 months, the overall survival rate at 20 months was 73.8% while the progression-free survival rate at 20 months was 24.2%. Globally nine patients are in continuous complete response with a median follow up of 14 months (range, 10-19 months). Four patients proceeded to autotransplantation and nine to allotransplantation. The most frequent extra-hematologic toxicity was peripheral neuropathy, observed in 21.5% of cases (9 patients with grade 1/2 and 5 patients with grade 3/4); neurological toxicity led to discontinuation of treatment in three patients and to dose reduction in four. In general the treatment was well tolerated and toxicities, both hematologic and extra-hematologic, were manageable. This report indicates and confirms that brentuximab vedotin as a single agent is effective and safe also when used in standard, everyday clinical practice outside a clinical trial. Best overall responses were recorded after three or four cycles and showed that brentuximab vedotin provides an effective bridge to further therapeutic interventions.
Figures


Similar articles
-
Brentuximab vedotin plus bendamustine in relapsed or refractory Hodgkin's lymphoma: an international, multicentre, single-arm, phase 1-2 trial.Lancet Oncol. 2018 Feb;19(2):257-266. doi: 10.1016/S1470-2045(17)30912-9. Epub 2017 Dec 21. Lancet Oncol. 2018. PMID: 29276022 Free PMC article. Clinical Trial.
-
Safety and efficacy of single-agent bendamustine after failure of brentuximab vedotin in patients with relapsed or refractory hodgkin's lymphoma: experience with 27 patients.Clin Lymphoma Myeloma Leuk. 2015 Jul;15(7):404-8. doi: 10.1016/j.clml.2015.02.023. Epub 2015 Mar 5. Clin Lymphoma Myeloma Leuk. 2015. PMID: 25840816
-
Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial.Lancet. 2015 May 9;385(9980):1853-62. doi: 10.1016/S0140-6736(15)60165-9. Epub 2015 Mar 19. Lancet. 2015. PMID: 25796459 Clinical Trial.
-
Brentuximab vedotin desensitization in a patient with refractory Hodgkin's lymphoma.Eur J Haematol. 2015 Oct;95(4):361-4. doi: 10.1111/ejh.12570. Epub 2015 May 6. Eur J Haematol. 2015. PMID: 25892213 Review.
-
Where does brentuximab vedotin fit into the management of patients with Hodgkin lymphoma?Curr Hematol Malig Rep. 2012 Sep;7(3):179-85. doi: 10.1007/s11899-012-0126-1. Curr Hematol Malig Rep. 2012. PMID: 22669711 Review.
Cited by
-
Durable Remission in Hodgkin Lymphoma Treated With One Cycle of Bleomycin, Vinblastine, Dacarbazine and Two Doses of Nivolumab and Brentuximab Vedotin.J Hematol. 2022 Aug;11(4):154-158. doi: 10.14740/jh1035. Epub 2022 Aug 30. J Hematol. 2022. PMID: 36118550 Free PMC article.
-
Immunotoxin - a new treatment option in patients with relapsed and refractory Hodgkin lymphoma.Radiol Oncol. 2015 Nov 27;49(4):315-9. doi: 10.1515/raon-2015-0036. eCollection 2015 Dec. Radiol Oncol. 2015. PMID: 26834516 Free PMC article. Review.
-
Infectious Complications of Targeted Therapies in Children with Leukemias and Lymphomas.Cancers (Basel). 2022 Oct 14;14(20):5022. doi: 10.3390/cancers14205022. Cancers (Basel). 2022. PMID: 36291806 Free PMC article. Review.
-
Brentuximab Vedotin in Transplant-Naïve Relapsed/Refractory Hodgkin Lymphoma: Experience in 30 Patients.Oncologist. 2015 Dec;20(12):1413-6. doi: 10.1634/theoncologist.2015-0227. Epub 2015 Oct 23. Oncologist. 2015. PMID: 26500229 Free PMC article.
-
FDG-PET/CT in the Monitoring of Lymphoma Immunotherapy Response: Current Status and Future Prospects.Cancers (Basel). 2023 Feb 7;15(4):1063. doi: 10.3390/cancers15041063. Cancers (Basel). 2023. PMID: 36831405 Free PMC article. Review.
References
-
- Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, et al. Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327 (21):1478–84 - PubMed
-
- Diehl V, Franklin J, Pfreundschuh M, Lathan B, Paulus U, Hasenclever D, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP_ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003;348:(7)2386–95 - PubMed
-
- Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin’s disease: results of a BNLI randomised trial. Lancet. 1993;341(8852): 1051–4 - PubMed
-
- Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haematopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065–71 - PubMed
-
- Andre M, Henry-Amar M, Pico JL, Brice P, Blaise D, Kuentz M, et al. Comparison of high-dose therapy and autologous stem-cell transplantation with conventional therapy for Hodgkin’s disease induction failure: A case-control study. J Clin Oncol. 1999;17 (1):222–9 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

