Cell-free hemoglobin and its scavenger proteins: new disease models leading the way to targeted therapies
- PMID: 23645855
- PMCID: PMC3662353
- DOI: 10.1101/cshperspect.a013433
Cell-free hemoglobin and its scavenger proteins: new disease models leading the way to targeted therapies
Abstract
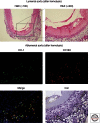
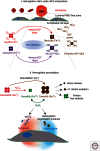
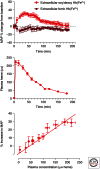
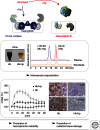
Hemoglobin (Hb) has multiple pathophysiologic effects when released into the intravascular space during hemolysis. The extracellular effects of Hb have resulted in novel models of toxicity, which help to explain endothelial dysfunction and cardiovascular complications that accompany genetic hemolytic anemias, malaria, blood transfusion, and atherosclerosis. The majority of models focus on nitric oxide (NO) depletion; however, in local tissue environments, Hb can also act as a pro-oxidant and inflammatory agent. This can alter cellular differentiation with the potential to deviate immune responses. The understanding of these mechanisms set in the context of natural scavenger and detoxification systems may accelerate the development of novel treatment strategies.
Figures

References
-
- Abraham NG, Kappas A 2008. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 60: 79–127 - PubMed
-
- Alayash AI 2004. Oxygen therapeutics: Can we tame haemoglobin? Nat Rev Drug Discov 3: 152–159 - PubMed
-
- Baldwin AL, Wiley EB, Alayash AI 2002. Comparison of effects of two hemoglobin-based O(2) carriers on intestinal integrity and microvascular leakage. Am J Physiol Heart Circ Physiol 283: H1292–H1301 - PubMed
-
- Baldwin AL, Wiley EB, Summers AG, Alayash AI 2003. Sodium selenite reduces hemoglobin-induced venular leakage in the rat mesentery. Am J Physiol Heart Circ Physiol 284: H81–H91 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
