A critical analysis of the clinical use of incretin-based therapies: Are the GLP-1 therapies safe?
- PMID: 23645885
- PMCID: PMC3687282
- DOI: 10.2337/dc12-2713
A critical analysis of the clinical use of incretin-based therapies: Are the GLP-1 therapies safe?
Abstract
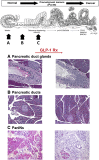
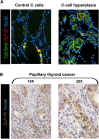
There is no question that incretin-based glucose-lowering medications have proven to be effective glucose-lowering agents. Glucagon-like peptide 1 (GLP-1) receptor agonists demonstrate an efficacy comparable to insulin treatment and appear to do so with significant effects to promote weight loss with minimal hypoglycemia. In addition, there are significant data with dipeptidyl peptidase 4 (DPP-4) inhibitors showing efficacy comparable to sulfonylureas but with weight neutral effects and reduced risk for hypoglycemia. However, over the recent past there have been concerns reported regarding the long-term consequences of using such therapies, and the issues raised are in regard to the potential of both classes to promote acute pancreatitis, to initiate histological changes suggesting chronic pancreatitis including associated preneoplastic lesions, and potentially, in the long run, pancreatic cancer. Other issues relate to a potential risk for the increase in thyroid cancer. There are clearly conflicting data that have been presented in preclinical studies and in epidemiologic studies. To provide an understanding of both sides of the argument, we provide a discussion of this topic as part of this two-part point-counterpoint narrative. In the point narrative below, Dr. Butler and colleagues provide their opinion and review of the data to date and that we need to reconsider the use of incretin-based therapies because of the growing concern of potential risk and based on a clearer understanding of the mechanism of action. In the counterpoint narrative following the contribution by Dr. Butler and colleagues, Dr. Nauck provides a defense of incretin-based therapies and that the benefits clearly outweigh any concern of risk.
Figures
Comment in
-
A critical analysis of the clinical use of incretin-based therapies: The benefits by far outweigh the potential risks.Diabetes Care. 2013 Jul;36(7):2126-32. doi: 10.2337/dc12-2504. Epub 2013 May 3. Diabetes Care. 2013. PMID: 23645884 Free PMC article.
-
Comment on: Butler et al. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care 2013;36:2118-2125.Diabetes Care. 2013 Dec;36(12):e213. doi: 10.2337/dc13-1134. Diabetes Care. 2013. PMID: 24265382 Free PMC article. No abstract available.
-
Response to comment on: Butler et al. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care 2013;36:2118-2125.Diabetes Care. 2013 Dec;36(12):e214. doi: 10.2337/dc13-1542. Diabetes Care. 2013. PMID: 24265383 Free PMC article. No abstract available.
References
-
- Gale EAM. Collateral damage: the conundrum of drug safety. Diabetologia 2009;52:1975–1982 - PubMed
-
- Dore DD, Bloomgren GL, Wenten M, et al. A cohort study of acute pancreatitis in relation to exenatide use. Diabetes Obes Metab 2011;13:559–566 - PubMed
-
- Dore DD, Seeger JD, Arnold Chan K. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin 2009;25:1019–1027 - PubMed
-
- Girman CJ, Kou TD, Cai B, et al. Patients with type 2 diabetes mellitus have higher risk for acute pancreatitis compared with those without diabetes. Diabetes Obes Metab 2010;12:766–771 - PubMed
-
- Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology 2000;141:4600–4605 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

