Novel insights on thyroid-stimulating hormone receptor signal transduction
- PMID: 23645907
- PMCID: PMC3785642
- DOI: 10.1210/er.2012-1072
Novel insights on thyroid-stimulating hormone receptor signal transduction
Abstract
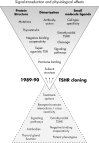
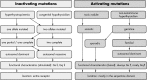
The TSH receptor (TSHR) is a member of the glycoprotein hormone receptors, a subfamily of family A G protein-coupled receptors. The TSHR is of great importance for the growth and function of the thyroid gland. The TSHR and its endogenous ligand TSH are pivotal proteins with respect to a variety of physiological functions and malfunctions. The molecular events of TSHR regulation can be summarized as a process of signal transduction, including signal reception, conversion, and amplification. The steps during signal transduction from the extra- to the intracellular sites of the cell are not yet comprehensively understood. However, essential new insights have been achieved in recent years on the interrelated mechanisms at the extracellular region, the transmembrane domain, and intracellular components. This review contains a critical summary of available knowledge of the molecular mechanisms of signal transduction at the TSHR, for example, the key amino acids involved in hormone binding or in the structural conformational changes that lead to G protein activation or signaling regulation. Aspects of TSHR oligomerization, signaling promiscuity, signaling selectivity, phenotypes of genetic variations, and potential extrathyroidal receptor activity are also considered, because these are relevant to an understanding of the overall function of the TSHR, including physiological, pathophysiological, and pharmacological perspectives. Directions for future research are discussed.
Figures



References
-
- Vassart G, Pardo L, Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem Sci. 2004;29:119–126 - PubMed
-
- Allgeier A, Offermanns S, Van Sande J, Spicher K, Schultz G, Dumont JE. The human thyrotropin receptor activates G-proteins Gs and Gq/11. J Biol Chem. 1994;269:13733–13735 - PubMed
-
- Laurent E, Mockel J, Van Sande J, Graff I, Dumont JE. Dual activation by thyrotropin of the phospholipase C and cyclic AMP cascades in human thyroid. Mol Cell Endocrinol. 1987;52:273–278 - PubMed
-
- Van Sande J, Raspé E, Perret J, et al. Thyrotropin activates both the cyclic AMP and the PIP2 cascades in CHO cells expressing the human cDNA of TSH receptor. Mol Cell Endocrinol. 1990;74:R1–R6 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

