A synthetic podophyllotoxin derivative exerts anti-cancer effects by inducing mitotic arrest and pro-apoptotic ER stress in lung cancer preclinical models
- PMID: 23646116
- PMCID: PMC3639983
- DOI: 10.1371/journal.pone.0062082
A synthetic podophyllotoxin derivative exerts anti-cancer effects by inducing mitotic arrest and pro-apoptotic ER stress in lung cancer preclinical models
Abstract
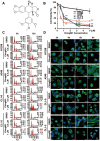
Some potent chemotherapy drugs including tubulin-binding agents had been developed from nature plants, such as podophyllotoxin and paclitaxel. However, poor cytotoxic selectivity, serious side-effects, and limited effectiveness are still the major concerns in their therapeutic application. We developed a fully synthetic podophyllotoxin derivative named Ching001 and investigated its anti-tumor growth effects and mechanisms in lung cancer preclinical models. Ching001 showed a selective cytotoxicity to different lung cancer cell lines but not to normal lung cells. Ching001 inhibited the polymerization of microtubule resulting in mitotic arrest as evident by the accumulation of mitosis-related proteins, survivin and aurora B, thereby leading to DNA damage and apoptosis. Ching001 also activated pro-apoptotic ER stress signaling pathway. Intraperitoneal injection of 2 mg/kg Ching001 significantly inhibited the tumor growth of A549 xenograft, while injection of 0.2 mg/kg Ching001 decreased the lung colonization ability of A549 cells in experimental metastasis assay. These anti-tumor growth and lung colonization inhibition effects were stronger than those of paclitaxel treatment at the same dosage. The xenograft tumor tissue stains further confirmed that Ching001 induced mitosis arrest and tumor apoptosis. In addition, the hematology and biochemistry tests of blood samples as well as tissue examinations indicated that Ching001 treatment did not show apparent organ toxicities in tested animals. We provided preclinical evidence that novel synthetic microtubule inhibitor Ching001, which can trigger DNA damage and apoptosis by inducing mitotic arrest and ER stress, is a potential anti-cancer compound for further drug development.
Conflict of interest statement
Figures






References
-
- Imbert TF (1998) Discovery of podophyllotoxins. Biochimie 80: 207–222. - PubMed
-
- Liu EH, Qi LW, Wu Q, Peng YB, Li P (2009) Anticancer agents derived from natural products. Mini Rev Med Chem 9: 1547–1555. - PubMed
-
- Lv M, Xu H (2011) Recent advances in semisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: an update (2008–2010). Mini Rev Med Chem 11: 901–909. - PubMed
-
- Komericki P, Akkilic-Materna M, Strimitzer T, Aberer W (2011) Efficacy and safety of imiquimod versus podophyllotoxin in the treatment of anogenital warts. Sex Transm Dis 38: 216–218. - PubMed
-
- Desbene S, Giorgi-Renault S (2002) Drugs that inhibit tubulin polymerization: the particular case of podophyllotoxin and analogues. Curr Med Chem Anticancer Agents 2: 71–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

