Antifungal activity of fused Mannich ketones triggers an oxidative stress response and is Cap1-dependent in Candida albicans
- PMID: 23646117
- PMCID: PMC3639977
- DOI: 10.1371/journal.pone.0062142
Antifungal activity of fused Mannich ketones triggers an oxidative stress response and is Cap1-dependent in Candida albicans
Abstract
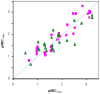
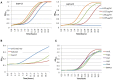
We investigated the antifungal activity of fused Mannich ketone (FMK) congeners and two of their aminoalcohol derivatives. In particular, FMKs with five-membered saturated rings were shown to have minimum inhibitory concentration (MIC90s) ranging from 0.8 to 6 µg/mL toward C. albicans and the closely related C. parapsilosis and C. krusei while having reduced efficacy toward C. glabrata and almost no efficacy against Aspergillus sp. Transcript profiling of C. albicans cells exposed for 30 or 60 min to 2-(morpholinomethyl)-1-indanone, a representative FMK with a five-membered saturated ring, revealed a transcriptional response typical of oxidative stress and similar to that of a C. albicans Cap1 transcriptional activator. Consistently, C. albicans lacking the CAP1 gene was hypersensitive to this FMK, while C. albicans strains overexpressing CAP1 had decreased sensitivity to 2-(morpholinomethyl)-1-indanone. Quantitative structure-activity relationship studies revealed a correlation of antifungal potency and the energy of the lowest unoccupied molecular orbital of FMKs and unsaturated Mannich ketones thereby implicating redox cycling-mediated oxidative stress as a mechanism of action. This conclusion was further supported by the loss of antifungal activity upon conversion of representative FMKs to aminoalcohols that were unable to participate in redox cycles.
Conflict of interest statement
Figures


References
-
- Pfaller MA, Diekema DJ (2010) Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 36: 1–53. - PubMed
-
- Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, Rex JH (2010) An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov 9: 719–727. - PubMed
-
- Pelletier SW, Venkov AP, Finermoore J, Modey NV (1980) Alumina Catalyzed Addition of Secondary-Amines to Exocyclic Alpha,Beta-Unsaturated Ketones. Tetrahedron Letters 21: 809–812.
-
- Zhao YJ, Wei W, Su ZG, Ma GH (2009) Poly (ethylene glycol) prodrug for anthracyclines via N-Mannich base linker: design, synthesis and biological evaluation. Int J Pharm 379: 90–99. - PubMed
-
- Bundgaard H, Johansen M, Stella V, Cortese M (1982) Pro-Drugs as Drug Delivery Systems.21. Preparation, Physicochemical Properties and Bioavailability of a Novel Water-Soluble Pro-Drug Type for Carbamazepine. International Journal of Pharmaceutics 10: 181–192.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

