Suppression of cholangiocarcinoma cell growth by human umbilical cord mesenchymal stem cells: a possible role of Wnt and Akt signaling
- PMID: 23646150
- PMCID: PMC3639969
- DOI: 10.1371/journal.pone.0062844
Suppression of cholangiocarcinoma cell growth by human umbilical cord mesenchymal stem cells: a possible role of Wnt and Akt signaling
Erratum in
- PLoS One. 2013;8(7). doi:10.1371/annotation/7fff93fa-8e80-41f3-b35a-e658cb7256a9
Abstract
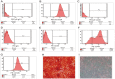
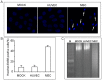
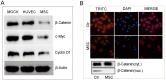
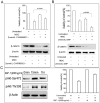
Emerging evidence indicates that human mesenchymal stem cells (hMSCs) can be recruited to tumor sites, and affect the growth of human malignancies. However, little is known about the underlying molecular mechanisms. Here, we observed the effects of hMSCs on the human cholangiocarcinoma cell line, HCCC-9810, using an animal transplantation model, and conditioned media from human umbilical cord-derived mesenchymal stem cells (hUC-MSCs). Animal studies showed that hUC-MSCs can inhibit the growth of cholangiocarcinoma xenograft tumors. In cell culture, conditioned media from hUC-MSCs inhibited proliferation and induced apoptosis of tumor cells in a dose- and time-dependent manner. The proliferation inhibition rate increased from 6.21% to 49.86%, whereas the apoptosis rate increased from 9.3% to 48.1% when HCCC-9810 cells were cultured with 50% hUC-MSC conditioned media for 24 h. Immunoblot analysis showed that the expression of phosphor-PDK1 (Ser241), phosphor-Akt (Ser 437 and Thr308), phosphorylated glycogen synthase kinase 3β (phospho-GSK-3β(Ser9)), β-catenin, cyclin-D1, and c-myc were down-regulated. We further demonstrated that CHIR99021, a GSK-3β inhibitor reversed the suppressive effects of hUC-MSCs on HCCC-9810 cells and increased the expression of β-catenin. The GSK-3β activator, sodium nitroprusside dehydrate (SNP), augmented the anti-tumor effects of hUC-MSCs and decreased the expression of β-catenin. IGF-1 acted as an Akt activator, and also reversed the suppressive effects of hUC-MSCs on HCCC-9810 cells. All these results suggest that hUC-MSCs could inhibit the malignant phenotype of HCCC-9810 human cholangiocarcinoma cell line. The cross-talk role of Wnt/β-catenin and PI3K/Akt signaling pathway, with GSK-3β as the key enzyme bridging these pathways, may contribute to the inhibition of cholangiocarcinoma cells by hUC-MSCs.
Conflict of interest statement
Figures



Similar articles
-
Involvement of Wnt/β-catenin signaling in the mesenchymal stem cells promote metastatic growth and chemoresistance of cholangiocarcinoma.Oncotarget. 2015 Dec 8;6(39):42276-89. doi: 10.18632/oncotarget.5514. Oncotarget. 2015. PMID: 26474277 Free PMC article.
-
Human umbilical cord mesenchymal stem cells inhibit C6 glioma growth via secretion of dickkopf-1 (DKK1).Mol Cell Biochem. 2014 Jan;385(1-2):277-86. doi: 10.1007/s11010-013-1836-y. Epub 2013 Oct 9. Mol Cell Biochem. 2014. PMID: 24104453
-
Human umbilical cord-derived mesenchymal stem cell transplantation supplemented with curcumin improves the outcomes of ischemic stroke via AKT/GSK-3β/β-TrCP/Nrf2 axis.J Neuroinflammation. 2023 Feb 24;20(1):49. doi: 10.1186/s12974-023-02738-5. J Neuroinflammation. 2023. PMID: 36829224 Free PMC article.
-
Wnt/β-catenin signaling as an emerging potential key pharmacological target in cholangiocarcinoma.Biosci Rep. 2020 Mar 27;40(3):BSR20193353. doi: 10.1042/BSR20193353. Biosci Rep. 2020. PMID: 32140709 Free PMC article. Review.
-
The duality of GSK-3β in urinary bladder cancer: Tumor suppressor and promoter roles through multiple signaling pathways.Biochim Biophys Acta Rev Cancer. 2025 Jul;1880(3):189324. doi: 10.1016/j.bbcan.2025.189324. Epub 2025 Apr 19. Biochim Biophys Acta Rev Cancer. 2025. PMID: 40258445 Review.
Cited by
-
Navigating Tumour Microenvironment and Wnt Signalling Crosstalk: Implications for Advanced Cancer Therapeutics.Cancers (Basel). 2023 Dec 14;15(24):5847. doi: 10.3390/cancers15245847. Cancers (Basel). 2023. PMID: 38136392 Free PMC article. Review.
-
Bidirectional and Opposite Effects of Naïve Mesenchymal Stem Cells on Tumor Growth and Progression.Adv Pharm Bull. 2019 Oct;9(4):539-558. doi: 10.15171/apb.2019.063. Epub 2019 Oct 24. Adv Pharm Bull. 2019. PMID: 31857958 Free PMC article. Review.
-
Activation of glycogen synthase kinase 3β ameliorates diabetes-induced kidney injury.J Biol Chem. 2014 Dec 19;289(51):35363-75. doi: 10.1074/jbc.M114.587840. Epub 2014 Oct 22. J Biol Chem. 2014. PMID: 25339176 Free PMC article.
-
Human chorion and placental mesenchymal stem cells conditioned media suppress cell migration and invasion by inhibiting the PI3K/AKT pathway in cholangiocarcinoma.Sci Rep. 2025 Aug 26;15(1):31472. doi: 10.1038/s41598-025-16789-6. Sci Rep. 2025. PMID: 40858767 Free PMC article.
-
Development and Characterization of Human Primary Cholangiocarcinoma Cell Lines.Am J Pathol. 2022 Sep;192(9):1200-1217. doi: 10.1016/j.ajpath.2022.05.007. Epub 2022 May 28. Am J Pathol. 2022. PMID: 35640676 Free PMC article. Review.
References
-
- Higashi M, Yamada N, Yokoyama S, Kitamoto S, Tabata K, et al. (2012) Pathobiological implications of MUC16/CA125 expression in intrahepatic cholangiocarcinoma-mass forming type. Pathobiology 79(2): 101–106. - PubMed
-
- Nathan H, Pawlik TM, Wolfgang CL, Choti MA, Cameron JL, et al. (2007) Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg 11: 1488–1496. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147. - PubMed
-
- Pittenger MF, Martin BJ (2004) Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res 95: 9–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

