Ablation of C/EBP homologous protein does not protect T17M RHO mice from retinal degeneration
- PMID: 23646198
- PMCID: PMC3640035
- DOI: 10.1371/journal.pone.0063205
Ablation of C/EBP homologous protein does not protect T17M RHO mice from retinal degeneration
Abstract
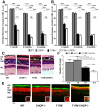
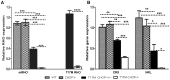
Despite the proposed link between ablation of the CHOP protein and delay of the onset of ER stress-mediated disorders including diabetes, Alzheimer Disease, and cardiac hypertrophy, the role of CHOP protein in photoreceptor cell death associated with Autosomal Dominant Retinitis Pigmentosa (ADRP) has not been investigated. T17M RHO transgenic mice carry a mutated human rhodopsin transgene, the expression of which in retina leads to protein misfolding, activation of UPR and progressive retinal degeneration. The purpose of this study is to investigate the role of CHOP protein in T17M RHO retina. Wild-type, CHOP-/-, T17M RHO and T17M RHO CHOP-/-mice were used in the study. Evaluation of the impact of CHOP ablation was performed using electroretinography (ERG), spectral-domain optical coherence tomography (SD-OCT), quantitative Real-Time PCR (qRT-PCR) and western blot analysis. Dark-adapted ERG analysis demonstrated that by 1 month, the T17M RHO CHOP-/- mice had a 70% reduction of the a-wave amplitude compared to the T17M RHO mice. The loss of function in T17M RHO CHOP-/- photoreceptors was associated with a 22-24% decline in the thickness of the outer nuclear layer. These mice had significant reduction in the expression of transcription factors, Crx and Nrl, and also in mouse Rho, and human RHO. The reduction was associated with an 8-fold elevation of the UPR marker, p-eIf2α protein and 30% down-regulation of sXbp1 protein. In addition, the histone deacetylase 1 (Hdac1) protein was 2-fold elevated in the T17M RHO CHOP-/- retina. The ablation of CHOP led to a reduction in the expression of photoreceptor-specific transcriptional factors, and both endogenous and exogenous RHO mRNA. Thus, despite its role in promoting apoptosis, CHOP protects rod photoreceptors carrying an ADRP mutation.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

