Efficacy and ligand bias at the μ-opioid receptor
- PMID: 23646826
- PMCID: PMC3724102
- DOI: 10.1111/bph.12222
Efficacy and ligand bias at the μ-opioid receptor
Abstract
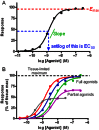
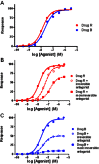
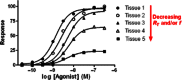
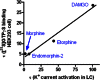
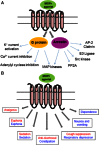
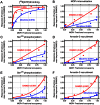
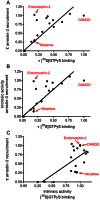
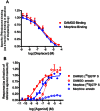
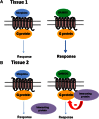
In order to describe drug action at a GPCR, a full understanding of the pharmacological terms affinity, efficacy and potency is necessary. This is true whether comparing the ability of different agonists to produce a measurable response in a cell or tissue, or determining the relative ability of an agonist to activate a single receptor subtype and produce multiple responses. There is a great deal of interest in the μ-opioid receptor (MOP receptor) and the ligands that act at this GPCR not only because of the clinically important analgesic effects produced by MOP agonists but also because of their liability to induce adverse effects such as respiratory depression and dependence. Our understanding of the mechanisms underlying these effects, as well as the ability to develop new, more effective MOP receptor drugs, depends upon the accurate determination of the efficacy with which these ligands induce coupling of MOP receptors to downstream signalling events. In this review, which is written with the minimum of mathematical content, the basic meaning of terms including efficacy, intrinsic activity and intrinsic efficacy is discussed, along with their relevance to the field of MOP receptor pharmacology, and in particular in relation to biased agonism at this important GPCR.
Keywords: GPCR; efficacy; intrinsic efficacy; ligand bias; μ-opioid receptor.
© 2013 The British Pharmacological Society.
Figures
References
-
- Bassoni DL, Raab WJ, Achacoso PL, Loh CY, Wehrman TS. Measurements of β-arrestin recruitment to activated seven transmembrane receptors using enzyme complementation. Methods Mol Biol. 2012;897:181–203. - PubMed
-
- Bennett MI, Graham J, Schmidt-Hansen M, Prettyjohns M, Arnold S, Group GD. Prescribing strong opioids for pain in adult palliative care: summary of NICE guidance. BMJ. 2012;344:e2806. - PubMed
-
- Black JW, Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci. 1983;220:141–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

