Integrated stress response of Escherichia coli to methylglyoxal: transcriptional readthrough from the nemRA operon enhances protection through increased expression of glyoxalase I
- PMID: 23646895
- PMCID: PMC3739934
- DOI: 10.1111/mmi.12234
Integrated stress response of Escherichia coli to methylglyoxal: transcriptional readthrough from the nemRA operon enhances protection through increased expression of glyoxalase I
Abstract
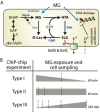
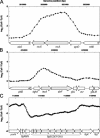
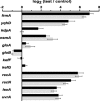
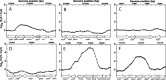
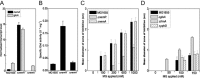
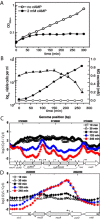
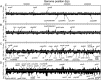
Methylglyoxal (MG) elicits activation of K(+) efflux systems to protect cells against the toxicity of the electrophile. ChIP-chip targeting RNA polymerase, supported by a range of other biochemical measurements and mutant creation, was used to identify genes transcribed in response to MG and which complement this rapid response. The SOS DNA repair regulon is induced at cytotoxic levels of MG, even when exposure to MG is transient. Glyoxalase I alone among the core MG protective systems is induced in response to MG exposure. Increased expression is an indirect consequence of induction of the upstream nemRA operon, encoding an enzyme system that itself does not contribute to MG detoxification. Moreover, this induction, via nemRA only occurs when cells are exposed to growth inhibitory concentrations of MG. We show that the kdpFABCDE genes are induced and that this expression occurs as a result of depletion of cytoplasmic K(+) consequent upon activation of the KefGB K(+) efflux system. Finally, our analysis suggests that the transcriptional changes in response to MG are a culmination of the damage to DNA and proteins, but that some integrate specific functions, such as DNA repair, to augment the allosteric activation of the main protective system, KefGB.
© 2013 The Authors. Molecular Microbiology published by John Wiley & Sons Ltd.
Figures


References
-
- Aldini G, Facino RM, Beretta G, Carini M. Carnosine and related dipeptides as quenchers of reactive carbonyl species: from structural studies to therapeutic perspectives. Biofactors. 2005;24:77–87. - PubMed
-
- Almeida Cd. 2009. Modelling of the protection mechanisms against methylgyoxal stress in Escherichia coli [electronic resource]: dynamical analysis and experimental validation. University of Aberdeen.
-
- Barnes WM, Tuley E. DNA sequence changes of mutations in the histidine operon control region that decrease attenuation. J Mol Biol. 1983;165:443–459. - PubMed
-
- Booth IR, Edwards MD, Miller S. Bacterial ion channels. Biochemistry. 2003;42:10045–10053. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

