A highly stereoselective addition of lithiated ynamides to Ellman-Davis chiral N-tert-butanesulfinyl imines
- PMID: 23646900
- PMCID: PMC3759603
- DOI: 10.1021/ol400989x
A highly stereoselective addition of lithiated ynamides to Ellman-Davis chiral N-tert-butanesulfinyl imines
Abstract
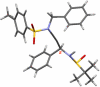
A highly diastereoselective addition of lithiated ynamides to Ellman-Davis chiral imines is described. While additions of N-sulfonyl ynamides are highly stereoselective even without Lewis acids, the use of BF3-OEt2 completely reversed the stereoselectivity. In addition, oxazolidinone-substituted ynamides behaved differently and functioned better with BF3-OEt2, and the chirality of the oxazolidinone ring exerts no impact on the selectivity.
Figures
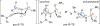
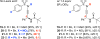


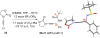

References
-
-
For current leading reviews on ynamides, see DeKorver KA, Li H, Lohse AG, Hayashi R, Lu Z, Zhang Y, Hsung RP. Chem. Rev. 2010;110:5064.Evano G, Coste A, Jouvin K. Angew. Chem. Int. Ed. 2010;49:2840.
-
-
-
For reviews partially accounting chemistry of ynamides, see: Ackermann L, Potukuchi HK. Org. Biomol. Chem. 2010;8:4503.Domínguez G, Perez-Castells J. Chem. Soc. Rev. 2011;40:3430.Weding N, Hapke M. Chem. Soc. Rev. 2011;40:4525.Madelaine C, Valerio V, Maulide N. Chem. Asian J. 2011;6:2224.
-
-
-
Given the volume, for ynamide chemistry published since September of 2012, see: Karmakar R, Mamidipalli P, Yun SY, Lee D. Org. Lett. 2013;15:1938.Brioche J, Meyer C, Cossy J. Org. Lett. 2013;15:1626.Yun SY, Wang K-P, Lee N-K, Mamidipalli P, Lee D. J. Am. Chem. Soc. 2013;135:4668.Heffernan SJ, Beddoes JM, Mahon MF, Hennessy AJ, Carbery DR. Chem. Commun. 2013;49:2314.Kong Y, Jiang K, Cao J, Fu L, Yu L, Lai G, Cui Y, Hu Z, Wang G. Org. Lett. 2013;15:422.Yavari I, Nematpour M, Sodagar E. Synlett. 2013;24:161.Yavari I, Nematpour M. Synlett. 2013;24:165.Bhunia S, Chang C-J, Liu R-S. Org. Lett. 2012;14:5522.Minko Y, Pasco M, Lercher L, Botoshansky M, Marek I. Nature. 2012;490:522.Hoye TR, Baire B, Niu D, Willoughby PH, Woods BP. Nature. 2012;490:208.Cao J, Kong Y, Deng Y, Lai G, Cui Y, Hu Z, Wang G. Org. Biomol. Chem. 2012;10:9556.Greenaway RL, Campbell CD, Chapman HA, Anderson EA. Adv. Synth. Catal. 2012;354:3187.Jiang Z, Lu P, Wang Y. Org. Lett. 2012;14:6266.Gati W, Rammah MM, Rammah MB, Evano G, Beilstein J. Org. Chem. 2012;8:2214.Smith DL, Chidipudi SR, Goundry WR, Lam HW. Org. Lett. 2012;14:4934.Heffernan SJ, Carbery DR. Tetrahedron Lett. 2012;53:5180.
-
-
-
For reviews on syntheses of ynamides, see: Mulder JA, Kurtz KCM, Hsung RP. Synlett. 2003:1379.Dehli JR, Legros J, Bolm C. Chem. Commun. 2005;43:973.Tracey MR, Hsung RP, Antoline JA, Kurtz KCM, Shen L, Slafer BW, Zhang Y. In: Science of Synthesis, Houben-Weyl Methods of Molecular Transformations. Weinreb Steve M., editor. Georg Thieme Verlag KG; Stuttgart, Germany: p. 2005. Chapter 21.4.Evano G, Blanchard N, Mathieu Toumi M. Chem. Rev. 2008;108:3054.Evano G, Jouvinb K, Coste A. Synthesis. 2013:17.
-
-
-
For syntheses of ynamides published in 2012, see: Jin X, Yamaguchi K, Mizuno N. Chem. Lett. 2012;41:866.Jin X, Yamaguchi K, Mizuno N. Chem. Commun. 2012;48:4974.Jouvin K, Heimburger J, Evano G. Chem. Sci. 2012;3:756.Jouvin K, Coste A, Bayle A, Legrand F, Karthikeyan G, Tadiparthi K, Evano G. Organometallics. 2012;31:7933.Tong X, Ni G, Deng X, Xia C. Synlett. 2012;23:2497.Wang M-G, Wu J, Shang Z-C. Synlett. 2012;23:589.Laouiti A, Jouvin K, Bourdreux F, Rammah MM, Rammah MB, Evano G. Synthesis. 2012;44:1491.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

