Imaging DNA with fluorochrome bearing metals
- PMID: 23646914
- PMCID: PMC3818342
- DOI: 10.1021/ic400404g
Imaging DNA with fluorochrome bearing metals
Abstract
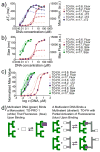
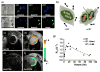
Molecules that fluoresce upon binding DNA are widely used in assaying and visualizing DNA in cells and tissues. However, using light to visualize DNA in animals is limited by the attenuation of light transmission by biological tissues. Moreover, it is now clear that DNA is an important mediator of dead cell clearance, coagulation reactions, and an immunogen in autoimmune lupus. Attaching metals (e.g., superparamagnetic nanoparticles, gadolinium ions, radioactive metal ions) to DNA-binding fluorochromes provides a way of imaging DNA in whole animals, and potentially humans, without light. Imaging metal-bearing, DNA-binding fluorochromes and their target DNA by magnetic resonance imaging may shed light on the many key roles of DNA in health and disease beyond the storage of genetic information.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Fluorochrome-functionalized magnetic nanoparticles for high-sensitivity monitoring of the polymerase chain reaction by magnetic resonance.Angew Chem Int Ed Engl. 2012 Jul 9;51(28):6904-7. doi: 10.1002/anie.201201661. Epub 2012 Jun 8. Angew Chem Int Ed Engl. 2012. PMID: 22684680 Free PMC article.
-
Modular Synthesis of Peptide-Based Single- and Multimodal Targeted Molecular Imaging Agents.ACS Appl Bio Mater. 2021 Jul 19;4(7):5435-5448. doi: 10.1021/acsabm.1c00157. Epub 2021 Jun 4. ACS Appl Bio Mater. 2021. PMID: 35006725 Free PMC article.
-
Fluorochrome-functionalized nanoparticles for imaging DNA in biological systems.ACS Nano. 2013 Mar 26;7(3):2032-41. doi: 10.1021/nn305962n. Epub 2013 Feb 7. ACS Nano. 2013. PMID: 23373524 Free PMC article.
-
Multimodal radio- (PET/SPECT) and fluorescence imaging agents based on metallo-radioisotopes: current applications and prospects for development of new agents.Dalton Trans. 2011 Jun 21;40(23):6129-43. doi: 10.1039/c0dt01398f. Epub 2011 Jan 12. Dalton Trans. 2011. PMID: 21225080 Review.
-
Gadolinium oxide nanoparticles as potential multimodal imaging and therapeutic agents.Curr Top Med Chem. 2013;13(4):422-33. doi: 10.2174/1568026611313040003. Curr Top Med Chem. 2013. PMID: 23432005 Review.
Cited by
-
Acute Cardiac Unloading and Recovery: Proceedings of the 4th Annual Acute Cardiac Unloading and REcovery (A-CURE) symposium held on 30 August 2019 in Paris, France.Interv Cardiol. 2019 Nov 18;14(3 Suppl 2):1-24. doi: 10.15420/icr.2019.14.3.S2. eCollection 2019 Nov. Interv Cardiol. 2019. PMID: 31969935 Free PMC article. No abstract available.
-
Design and Evaluation of Rhein-Based MRI Contrast Agents for Visualization of Tumor Necrosis Induced by Combretastatin A-4 Disodium Phosphate.Mol Imaging Biol. 2021 Apr;23(2):220-229. doi: 10.1007/s11307-020-01551-3. Epub 2020 Oct 13. Mol Imaging Biol. 2021. PMID: 33048270
References
-
- Pisetsky DS, Fairhurst AM. Autoimmunity. 2007;40:281–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

