Polysulfides link H2S to protein thiol oxidation
- PMID: 23646934
- PMCID: PMC3837443
- DOI: 10.1089/ars.2012.5041
Polysulfides link H2S to protein thiol oxidation
Abstract
Aims: Hydrogen sulfide (H2S) is suggested to act as a gaseous signaling molecule in a variety of physiological processes. Its molecular mechanism of action was proposed to involve protein S-sulfhydration, that is, conversion of cysteinyl thiolates (Cys-S(-)) to persulfides (Cys-S-S(-)). A central and unresolved question is how H2S-that is, a molecule with sulfur in its lowest possible oxidation state (-2)-can lead to oxidative thiol modifications.
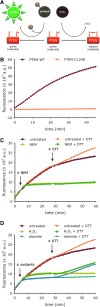
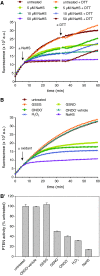
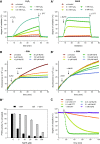
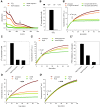
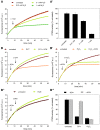
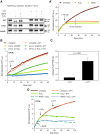
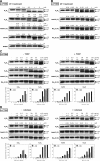
Results: Using the lipid phosphatase PTEN as a model protein, we find that the "H2S donor" sodium hydrosulfide (NaHS) leads to very rapid reversible oxidation of the enzyme in vitro. We identify polysulfides formed in NaHS solutions as the oxidizing species, and present evidence that sulfane sulfur is added to the active site cysteine. Polysulfide-mediated oxidation of PTEN was induced by all "H2S donors" tested, including sodium sulfide (Na2S), gaseous H2S, and morpholin-4-ium 4-methoxyphenyl(morpholino) phosphinodithioate (GYY4137). Moreover, we show that polysulfides formed in H2S solutions readily modify PTEN inside intact cells.
Innovation: Our results shed light on the previously unresolved question of how H2S leads to protein thiol oxidation, and suggest that polysulfides formed in solutions of H2S mediate this process.
Conclusion: This study suggests that the effects that have been attributed to H2S in previous reports may in fact have been mediated by polysulfides. It also supports the notion that sulfane sulfur rather than sulfide is the actual in vivo agent of H2S signaling.
Figures
Similar articles
-
Real-time assays for monitoring the influence of sulfide and sulfane sulfur species on protein thiol redox states.Methods Enzymol. 2015;555:57-77. doi: 10.1016/bs.mie.2014.11.020. Epub 2015 Jan 10. Methods Enzymol. 2015. PMID: 25747475
-
Effects of sulfane sulfur content in benzyl polysulfides on thiol-triggered H2S release and cell proliferation.Free Radic Biol Med. 2019 Feb 1;131:393-398. doi: 10.1016/j.freeradbiomed.2018.12.025. Epub 2018 Dec 21. Free Radic Biol Med. 2019. PMID: 30579781 Free PMC article.
-
Biological Effects of Morpholin-4-Ium 4 Methoxyphenyl (Morpholino) Phosphinodithioate and Other Phosphorothioate-Based Hydrogen Sulfide Donors.Antioxid Redox Signal. 2020 Jan 10;32(2):145-158. doi: 10.1089/ars.2019.7896. Antioxid Redox Signal. 2020. PMID: 31642346 Review.
-
Computational Study of H2S Release in Reactions of Diallyl Polysulfides with Thiols.J Phys Chem B. 2017 Jul 6;121(26):6359-6366. doi: 10.1021/acs.jpcb.7b03683. Epub 2017 Jun 23. J Phys Chem B. 2017. PMID: 28609097
-
GYY4137, a novel water-soluble, H2S-releasing molecule.Methods Enzymol. 2015;554:143-67. doi: 10.1016/bs.mie.2014.11.014. Epub 2015 Jan 10. Methods Enzymol. 2015. PMID: 25725521 Review.
Cited by
-
Hydrogen sulfide inhibits amyloid formation.J Phys Chem B. 2015 Jan 29;119(4):1265-74. doi: 10.1021/jp508471v. Epub 2015 Jan 15. J Phys Chem B. 2015. PMID: 25545790 Free PMC article.
-
Thermal spring water drinking attenuates dextran-sulfate-sodium-induced colitis in mice.Inflammopharmacology. 2015 Feb;23(1):57-64. doi: 10.1007/s10787-014-0227-7. Epub 2015 Jan 6. Inflammopharmacology. 2015. PMID: 25556814
-
Chloride channels mediate sodium sulphide-induced relaxation in rat uteri.Br J Pharmacol. 2015 Jul;172(14):3671-86. doi: 10.1111/bph.13161. Epub 2015 May 15. Br J Pharmacol. 2015. PMID: 25857480 Free PMC article.
-
TRPA1 Ion Channel Determines Beneficial and Detrimental Effects of GYY4137 in Murine Serum-Transfer Arthritis.Front Pharmacol. 2019 Sep 4;10:964. doi: 10.3389/fphar.2019.00964. eCollection 2019. Front Pharmacol. 2019. PMID: 31551776 Free PMC article.
-
Hydrogen Sulfide Biology and Its Role in Cancer.Molecules. 2022 May 25;27(11):3389. doi: 10.3390/molecules27113389. Molecules. 2022. PMID: 35684331 Free PMC article. Review.
References
-
- Cai WJ. Wang MJ. Moore PK. Jin HM. Yao T. Zhu YC. The novel proangiogenic effect of hydrogen sulfide is dependent on Akt phosphorylation. Cardiovasc Res. 2007;76:29–40. - PubMed
-
- Chen KY. Morris JC. Kinetics of oxidation of aqueous sulfide by O2. Environ Sci Technol. 1972;6:529.
-
- Coletta C. Papapetropoulos A. Erdelyi K. Olah G. Modis K. Panopoulos P. Asimakopoulou A. Gero D. Sharina I. Martin E. Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci U S A. 2012;109:9161–9166. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

