Distinct intrinsic and synaptic properties of pre-sympathetic and pre-parasympathetic output neurons in Barrington's nucleus
- PMID: 23647148
- PMCID: PMC3716857
- DOI: 10.1111/jnc.12290
Distinct intrinsic and synaptic properties of pre-sympathetic and pre-parasympathetic output neurons in Barrington's nucleus
Abstract
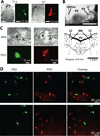
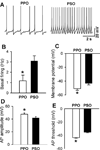
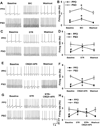
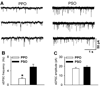
Barrington's nucleus (BN), commonly known as the pontine micturition center, controls micturition and other visceral functions through projections to the spinal cord. In this study, we developed a rat brain slice preparation to determine the intrinsic and synaptic mechanisms regulating pre-sympathetic output (PSO) and pre-parasympathetic output (PPO) neurons in the BN using patch-clamp recordings. The PSO and PPO neurons were retrogradely labeled by injecting fluorescent tracers into the intermediolateral region of the spinal cord at T13-L1 and S1-S2 levels, respectively. There were significantly more PPO than PSO neurons within the BN. The basal activity and membrane potential were significantly lower in PPO than in PSO neurons, and A-type K(+) currents were significantly larger in PPO than in PSO neurons. Blocking A-type K(+) channels increased the excitability more in PPO than in PSO neurons. Stimulting μ-opioid receptors inhibited firing in both PPO and PSO neurons. The glutamatergic EPSC frequency was much lower, whereas the glycinergic IPSC frequency was much higher, in PPO than in PSO neurons. Although blocking GABAA receptors increased the excitability of both PSO and PPO neurons, blocking glycine receptors increased the firing activity of PPO neurons only. Furthermore, blocking ionotropic glutamate receptors decreased the excitability of PSO neurons but paradoxically increased the firing activity of PPO neurons by reducing glycinergic input. Our findings indicate that the membrane and synaptic properties of PSO and PPO neurons in the BN are distinctly different. This information improves our understanding of the neural circuitry and central mechanisms regulating the bladder and other visceral organs.
Keywords: autonomic nervous system; micturition reflex; pontine micturition center; synaptic transmission; voltage-activated K+ channels.
© 2013 International Society for Neurochemistry.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Blok BF, Holstege G. Ultrastructural evidence for a direct pathway from the pontine micturition center to the parasympathetic preganglionic motoneurons of the bladder of the cat. Neurosci Lett. 1997;222:195–198. - PubMed
-
- Cano G, Card JP, Rinaman L, Sved AF. Connections of Barrington's nucleus to the sympathetic nervous system in rats. J Auton Nerv Syst. 2000;79:117–128. - PubMed
-
- de Groat WC, Booth AM, Yoshimura N. The Autonomic Nervous System. Nervous Control of the Urogenital System. In: Maggi CA, editor. Neurophysiology of micturition and its modification in animal models of human disease. Vol. 3. London: Hartwood Academic; 1993. pp. 227–289.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

