Improvement in middle cerebral artery structure and endothelial function in stroke-prone spontaneously hypertensive rats after macrophage depletion
- PMID: 23647512
- PMCID: PMC4603760
- DOI: 10.1111/micc.12064
Improvement in middle cerebral artery structure and endothelial function in stroke-prone spontaneously hypertensive rats after macrophage depletion
Abstract
Background: Inflammation is involved in the pathogenesis of hypertension. Hypertensive animals have an increased number of perivascular macrophages in cerebral arteries. Macrophages might be involved in remodeling of the cerebral vasculature. We hypothesized that peripheral macrophage depletion would improve MCA structure and function in hypertensive rats.
Methods: For macrophage depletion, six-week-old stroke-prone spontaneously hypertensive rats (SHRSP) were treated with CLOD, 10 mL/kg every three or four days, i.p., or vehicle (PBS lipo). MCA structure and function were analyzed by pressure and wire myography.
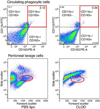
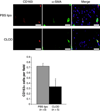
Results: Blood pressure was not affected by CLOD. The number of perivascular CD163-positive cells per microscopic field was reduced in the brain of SHRSP+CLOD. CLOD treatment caused an improvement in endothelium-dependent dilation after intralumenal perfusion of ADP and incubation with Ach. Inhibition of NO production blunted the Ach response, and endothelium-independent dilation was not altered. At an intralumenal pressure of 80 mmHg, MCA from SHRSP+CLOD showed increased lumen diameter, decreased wall thickness, and wall-to-lumen ratio. Cross-sectional area of pial arterioles from SHRSP+CLOD was higher than PBS lipo.
Conclusions: These results suggest that macrophage depletion attenuates MCA remodeling and improves MCA endothelial function in SHRSP.
Keywords: endothelium-dependent vasodilation; hypertension; liposome-encapsulated clodronate; middle cerebral artery; vascular remodeling.
© 2013 John Wiley & Sons Ltd.
Figures








Similar articles
-
Tumor necrosis factor-α inhibition attenuates middle cerebral artery remodeling but increases cerebral ischemic damage in hypertensive rats.Am J Physiol Heart Circ Physiol. 2014 Sep 1;307(5):H658-69. doi: 10.1152/ajpheart.00018.2014. Epub 2014 Jul 11. Am J Physiol Heart Circ Physiol. 2014. PMID: 25015967 Free PMC article.
-
Doxycycline, a matrix metalloprotease inhibitor, reduces vascular remodeling and damage after cerebral ischemia in stroke-prone spontaneously hypertensive rats.Am J Physiol Heart Circ Physiol. 2011 Jul;301(1):H87-97. doi: 10.1152/ajpheart.01206.2010. Epub 2011 May 6. Am J Physiol Heart Circ Physiol. 2011. PMID: 21551278 Free PMC article.
-
Tempol, a superoxide dismutase mimetic, prevents cerebral vessel remodeling in hypertensive rats.Microvasc Res. 2010 Dec;80(3):445-52. doi: 10.1016/j.mvr.2010.06.004. Epub 2010 Jun 22. Microvasc Res. 2010. PMID: 20600163 Free PMC article.
-
Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats.Microvasc Res. 2007 May;73(3):198-205. doi: 10.1016/j.mvr.2006.12.001. Epub 2007 Jan 23. Microvasc Res. 2007. PMID: 17250855 Free PMC article.
-
The effects of hypertension on the cerebral circulation.Am J Physiol Heart Circ Physiol. 2013 Jun 15;304(12):H1598-614. doi: 10.1152/ajpheart.00490.2012. Epub 2013 Apr 12. Am J Physiol Heart Circ Physiol. 2013. PMID: 23585139 Free PMC article. Review.
Cited by
-
Hypertension, dietary salt and cognitive impairment.J Cereb Blood Flow Metab. 2018 Dec;38(12):2112-2128. doi: 10.1177/0271678X18803374. Epub 2018 Oct 8. J Cereb Blood Flow Metab. 2018. PMID: 30295560 Free PMC article. Review.
-
Montelukast Ameliorates 2K1C-Hypertension Induced Endothelial Dysfunction and Associated Vascular Dementia.Curr Hypertens Rev. 2024;20(1):23-35. doi: 10.2174/0115734021276985231204092425. Curr Hypertens Rev. 2024. PMID: 38192137
-
Macrophages at CNS interfaces: ontogeny and function in health and disease.Nat Rev Neurosci. 2019 Sep;20(9):547-562. doi: 10.1038/s41583-019-0201-x. Epub 2019 Jul 29. Nat Rev Neurosci. 2019. PMID: 31358892 Review.
-
The immunological basis of hypertension.Am J Hypertens. 2014 Nov;27(11):1327-37. doi: 10.1093/ajh/hpu142. Epub 2014 Aug 23. Am J Hypertens. 2014. PMID: 25150828 Free PMC article. Review.
-
Brain perivascular macrophages: characterization and functional roles in health and disease.J Mol Med (Berl). 2017 Nov;95(11):1143-1152. doi: 10.1007/s00109-017-1573-x. Epub 2017 Aug 7. J Mol Med (Berl). 2017. PMID: 28782084 Free PMC article. Review.
References
-
- Aalkjaer C, Heagerty AM, Petersen KK, Swales JD, Mulvany MJ. Evidence for increased media thickness, increased neuronal amine uptake, and depressed excitation--contraction coupling in isolated resistance vessels from essential hypertensives. Circ Res. 1987;61:181–186. - PubMed
-
- Arenas IA, Xu Y, Lopez-Jaramillo P, Davidge ST. Angiotensin II-induced MMP-2 release from endothelial cells is mediated by TNF-alpha. Am J Physiol Cell Physiol. 2004;286:C779–C784. - PubMed
-
- Baumbach GL, Hajdu MA. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension. 1993;21:816–826. - PubMed
-
- Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

