Protein-L-isoaspartate (D-aspartate) O-methyltransferase protects cardiomyocytes against hypoxia induced apoptosis through inhibiting proapoptotic kinase Mst1
- PMID: 23647599
- PMCID: PMC3788851
- DOI: 10.1016/j.ijcard.2013.04.045
Protein-L-isoaspartate (D-aspartate) O-methyltransferase protects cardiomyocytes against hypoxia induced apoptosis through inhibiting proapoptotic kinase Mst1
Abstract
Background: Mammalian sterile 20-like kinase 1 (Mst1) is a mammalian homolog of Hippo kinase from Drosophila and it is a critical component of the Hippo signaling pathway, which regulates a variety of biological processes ranging from cell contact inhibition, organ size control, apoptosis and tumor suppression in mammals. Mst1 plays essential roles in heart disease since its activation causes cardiomyocyte apoptosis and dilated cardiomyopathy. However, the mechanism underlying Mst1 activation in the heart is not known.
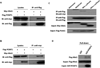
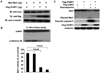
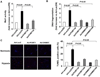
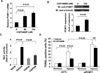
Methods and results: To identify novel cardiac proteins that may regulate Mst1 activity in the heart under pathophysiological conditions, a yeast two-hybrid screening of a human heart cDNA library with a dominant-negative Mst1 (K59R) mutant used as bait was performed. As a result, protein-L-isoaspartate (D-aspartate) O-methyltransferase (PCMT1) was identified as an Mst1-interacting protein. The interaction of PCMT1 with Mst1 was confirmed by co-immunoprecipitation in both co-transfected HEK293 cells and native cardiomyocytes, in which PCMT1 interacted with the kinase domain of Mst1, but not with its C-terminal regulatory domain. Overexpression of PCMT1 did not affect the Mst1 expression, but significantly attenuated the Mst1 activation and its apoptotic effects in response to the hypoxia/reoxygenation induced injury in cardiomyocytes. Indeed, upregulation of PCMT1 by CGP3466B, a compound related to the anti-Parkinson's drug R-(-)-deprenyl with potent antiapoptotic effects, inhibited the hypoxia/reoxygenation induced Mst1 activation and cardiomyocyte apoptosis.
Conclusions: These findings implicate PCMT1 as a novel inhibitor of Mst1 activation in cardiomyocytes and suggest that targeting PCMT1 may prevent myocardial apoptosis through inhibition of Mst1.
Keywords: Apoptosis; Cardiac myocytes; Hypoxia/reoxygenation; Mst1 kinase; PCMT1.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Potential conflicts of interest: none
Figures




Similar articles
-
Glyceraldehyde-3-phosphate dehydrogenase interacts with proapoptotic kinase mst1 to promote cardiomyocyte apoptosis.PLoS One. 2013;8(3):e58697. doi: 10.1371/journal.pone.0058697. Epub 2013 Mar 20. PLoS One. 2013. PMID: 23527007 Free PMC article.
-
Neuroprotective Effects of CGP3466B on Apoptosis Are Modulated by Protein-L-isoaspartate (D-aspartate) O-methyltransferase/Mst1 Pathways after Traumatic Brain Injury in Rats.Sci Rep. 2017 Aug 23;7(1):9201. doi: 10.1038/s41598-017-08196-3. Sci Rep. 2017. PMID: 28835703 Free PMC article.
-
PCMT1 Ameliorates Neuronal Apoptosis by Inhibiting the Activation of MST1 after Subarachnoid Hemorrhage in Rats.Transl Stroke Res. 2017 May 22. doi: 10.1007/s12975-017-0540-8. Online ahead of print. Transl Stroke Res. 2017. PMID: 28534197
-
Understanding the role of mammalian sterile 20-like kinase 1 (MST1) in cardiovascular disorders.J Mol Cell Cardiol. 2018 Jan;114:141-149. doi: 10.1016/j.yjmcc.2017.11.010. Epub 2017 Nov 15. J Mol Cell Cardiol. 2018. PMID: 29155025 Review.
-
MST1: A future novel target for cardiac diseases.Int J Biol Macromol. 2023 Jun 1;239:124296. doi: 10.1016/j.ijbiomac.2023.124296. Epub 2023 Apr 1. Int J Biol Macromol. 2023. PMID: 37011743 Review.
Cited by
-
PCMT1 regulates the migration, invasion, and apoptosis of prostate cancer through modulating the PI3K/AKT/GSK-3β pathway.Aging (Albany NY). 2023 Oct 27;15(20):11654-11671. doi: 10.18632/aging.205152. Epub 2023 Oct 27. Aging (Albany NY). 2023. PMID: 37899170 Free PMC article.
-
The Hippo pathway in disease and therapy: cancer and beyond.Clin Transl Med. 2014 Jul 10;3:22. doi: 10.1186/2001-1326-3-22. eCollection 2014. Clin Transl Med. 2014. PMID: 25097725 Free PMC article. Review.
-
Telomere Maintenance-Related Genes are Essential for Prognosis in Breast Cancer.Breast Cancer (Dove Med Press). 2025 Feb 24;17:225-239. doi: 10.2147/BCTT.S506783. eCollection 2025. Breast Cancer (Dove Med Press). 2025. PMID: 40028272 Free PMC article.
-
A Proof of Principle 2D Spatial Proteome Mapping Analysis Reveals Distinct Regional Differences in the Cardiac Proteome.Life (Basel). 2024 Aug 1;14(8):970. doi: 10.3390/life14080970. Life (Basel). 2024. PMID: 39202712 Free PMC article.
-
Short-Term Blockade of Pro-Inflammatory Alarmin S100A9 Favorably Modulates Left Ventricle Proteome and Related Signaling Pathways Involved in Post-Myocardial Infarction Recovery.Int J Mol Sci. 2022 May 9;23(9):5289. doi: 10.3390/ijms23095289. Int J Mol Sci. 2022. PMID: 35563680 Free PMC article.
References
-
- Chan SW, Lim CJ, Chen L, Chong YF, Huang C, Song H, et al. The hippo pathway in biological control and cancer development. J Cell Physiol. 2011;226:928–939. - PubMed
-
- Creasy CL, Ambrose DM, Chernoff J. The Ste20-like protein kinase, Mst1, dimerizes and contains an inhibitory domain. J Biol Chem. 1996;271:21049–21053. - PubMed
-
- Lin Y, Khokhlatchev A, Figeys D, Avruch J. Death-associated protein 4 binds MST1 and augments MST1-induced apoptosis. J Biol Chem. 2002;277:47991–48001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

