Simplified negative pressure wound therapy: clinical evaluation of an ultraportable, no-canister system
- PMID: 23647737
- PMCID: PMC7950749
- DOI: 10.1111/iwj.12080
Simplified negative pressure wound therapy: clinical evaluation of an ultraportable, no-canister system
Abstract
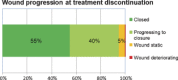
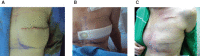
The aim of this study was to evaluate a prototype negative pressure wound therapy (NPWT) system that has been developed to simplify NPWT for wounds at the lower end of the acuity scale. The new device has a single preset pressure of -80 mmHg, is single use and operates without an exudate canister. The disposable NPWT system (PICO™) was tested in a prospective, non-comparative, multicentre clinical trial to assess device functionality and clinical acceptance. Twenty patients were recruited for a maximum treatment period of 14 days. The NPWT devices were fitted with data log chips to enable longitudinal assessment of negative pressure and leak rates during therapy. Sixteen (80%) patients had closed surgical wounds, two (10%) patients had traumatic wounds and two (10%) patients received meshed split thickness skin grafts. The mean study duration was 10·7 days (range: 5-14 days) and the mean dressing wear time per individual patient was 4·6 days (range: 2-11). Fifty-five percent of wounds had closed by the end of the 14-day study or earlier, with a further 40% of wounds progressing to closure. Real-time pressure monitoring showed continuous delivery of NPWT. Three cases are discussed representing different wound locations and different patient factors that can increase the risk of post-surgical complications. Clinical studies of the disposable NPWT system confirmed the ability of the simplified single-use device to function consistently over the expected wear time. The anticipated reduced costs, ease of use and increased mobility of patients using this system may enable NPWT benefits to be available to a greater proportion of patients.
Keywords: Closed surgical incision; Negative pressure wound therapy; Portable; Postoperative care; Wound healing.
© 2013 The Authors. International Wound Journal published by Medicalhelplines.com Inc and John Wiley & Sons Ltd.
Figures



Similar articles
-
Using low pressure, NPWT for wound preparation & the management of split-thickness skin grafts in 3 patients with complex wound.Ostomy Wound Manage. 2009 Jun 1;55(6):32-42. Ostomy Wound Manage. 2009. PMID: 19564671
-
Navigating new technologies in negative pressure wound therapy.Plast Surg Nurs. 2011 Apr-Jun;31(2):65-72; quiz 73-4. doi: 10.1097/PSN.0b013e318219778b. Plast Surg Nurs. 2011. PMID: 21633272
-
A new portable negative pressure wound therapy device: a prospective study investigating clinical outcomes.J Wound Care. 2024 Nov 2;33(11):833-840. doi: 10.12968/jowc.2024.0033. J Wound Care. 2024. PMID: 39480726
-
Negative pressure wound therapy: device design, indications, and the evidence supporting its use.Expert Rev Med Devices. 2021 Feb;18(2):151-160. doi: 10.1080/17434440.2021.1882301. Epub 2021 Mar 12. Expert Rev Med Devices. 2021. PMID: 33496626 Review.
-
EWMA Document: Negative Pressure Wound Therapy.J Wound Care. 2017 Mar 1;26(Sup3):S1-S154. doi: 10.12968/jowc.2017.26.Sup3.S1. J Wound Care. 2017. PMID: 28345371 Review.
Cited by
-
Skin grafting treatment of adolescent lower limb avulsion injury.Front Surg. 2022 Sep 15;9:953038. doi: 10.3389/fsurg.2022.953038. eCollection 2022. Front Surg. 2022. PMID: 36189402 Free PMC article. Review.
-
INCISIONAL NEGATIVE-PRESSURE WOUND THERAPY IN REVISION TOTAL HIP ARTHROPLASTY DUE TO INFECTION.Acta Ortop Bras. 2018;26(5):300-304. doi: 10.1590/1413-785220182605196038. Acta Ortop Bras. 2018. PMID: 30464709 Free PMC article.
-
Negative pressure wound therapy for managementof the surgical incision in orthopaedic surgery: A review of evidence and mechanisms for an emerging indication.Bone Joint Res. 2013 Dec 18;2(12):276-84. doi: 10.1302/2046-3758.212.2000190. Print 2013. Bone Joint Res. 2013. PMID: 24352756 Free PMC article.
-
Moist Wound Healing with Commonly Available Dressings.Adv Wound Care (New Rochelle). 2021 Dec;10(12):685-698. doi: 10.1089/wound.2020.1232. Epub 2021 Feb 11. Adv Wound Care (New Rochelle). 2021. PMID: 32870777 Free PMC article.
-
Single use negative-pressure wound therapy compared to standard care in patients after carbon dioxide laser surgery for hidradenitis suppurativa.Dermatol Ther. 2022 Jun;35(6):e15483. doi: 10.1111/dth.15483. Epub 2022 Apr 6. Dermatol Ther. 2022. PMID: 35373495 Free PMC article.
References
-
- Armstrong DG, Lavery LA. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet 2005;366:1704–10. - PubMed
-
- Wanner MB, Schwarzl F, Strub B, Zaech GA, Pierer G. Vacuum‐assisted wound closure for cheaper and more comfortable healing of pressure sores: a prospective study. Scand J Plast Reconstr Surg Hand Surg 2003;37:28–33. - PubMed
-
- Vuerstaek JDD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State‐of‐the‐art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum‐assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006;44:1029–37, discussion 1038. - PubMed
-
- Kamolz L‐P, Andel H, Haslik W, Winter W, Meissl G, Frey M. Use of subatmospheric pressure therapy to prevent burn wound progression in human: first experiences. Burns 2004;30:253–8. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

