Targeting unfolded protein response signaling pathways to ameliorate protein misfolding diseases
- PMID: 23647985
- PMCID: PMC5859939
- DOI: 10.1016/j.cbpa.2013.04.009
Targeting unfolded protein response signaling pathways to ameliorate protein misfolding diseases
Abstract
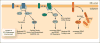
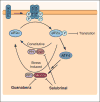
Protein homeostasis (or proteostasis) within the endoplasmic reticulum (ER) is regulated by the unfolded protein response (UPR). The UPR consists of three integrated signaling pathways activated by the accumulation of misfolded proteins within the ER lumen. Activation of the UPR alters ER proteostasis through translational attenuation of new protein synthesis and transcriptional remodeling of ER proteostasis pathways, providing a mechanism to adapt ER proteostasis in response to cellular stress. The capacity of the UPR to alter ER proteostasis suggests that exogenous manipulation of UPR signaling pathways offers therapeutic promise to alter the fate of pathologic proteins associated with human protein misfolding diseases. Here, we discuss the therapeutic potential of exogenous UPR activation to treat human disease and highlight specific small molecule approaches for regulating UPR signaling that could be beneficial to treat protein misfolding diseases.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

References
-
- Braakman I, Bulleid NJ. Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem. 2011;80:71–99. - PubMed
-
- Wiseman RL, et al. An adaptable standard for protein export from the endoplasmic reticulum. Cell. 2007;131:809–821. - PubMed
-
- Powers ET, et al. Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem. 2009;78:959–991. - PubMed
-
- Balch WE, et al. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

