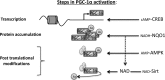
The protein level of PGC-1α, a key metabolic regulator, is controlled by NADH-NQO1
- PMID: 23648480
- PMCID: PMC3700121
- DOI: 10.1128/MCB.01672-12
The protein level of PGC-1α, a key metabolic regulator, is controlled by NADH-NQO1
Abstract
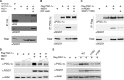
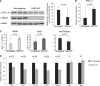
PGC-1α is a key transcription coactivator regulating energy metabolism in a tissue-specific manner. PGC-1α expression is tightly regulated, it is a highly labile protein, and it interacts with various proteins--the known attributes of intrinsically disordered proteins (IDPs). In this study, we characterize PGC-1α as an IDP and demonstrate that it is susceptible to 20S proteasomal degradation by default. We further demonstrate that PGC-1α degradation is inhibited by NQO1, a 20S gatekeeper protein. NQO1 binds and protects PGC-1α from degradation in an NADH-dependent manner. Using different cellular physiological settings, we also demonstrate that NQO1-mediated PGC-1α protection plays an important role in controlling both basal and physiologically induced PGC-1α protein level and activity. Our findings link NQO1, a cellular redox sensor, to the metabolite-sensing network that tunes PGC-1α expression and activity in regulating energy metabolism.
Figures





References
-
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, Kelly DP, Holloszy JO. 2002. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 16:1879–1886 - PubMed
-
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. 1999. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98:115–124 - PubMed
-
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. 2001. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413:179–183 - PubMed
-
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. 2001. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413:131–138 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
