The LIM-Homeodomain transcription factor Islet-1 is required for the development of sympathetic neurons and adrenal chromaffin cells
- PMID: 23648511
- PMCID: PMC5544970
- DOI: 10.1016/j.ydbio.2013.04.027
The LIM-Homeodomain transcription factor Islet-1 is required for the development of sympathetic neurons and adrenal chromaffin cells
Abstract
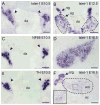
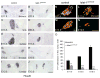
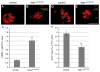
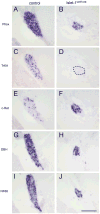
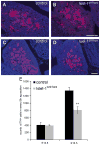
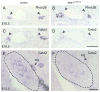
Islet-1 is a LIM-Homeodomain transcription factor with important functions for the development of distinct neuronal and non-neuronal cell populations. We show here that Islet-1 acts genetically downstream of Phox2B in cells of the sympathoadrenal cell lineage and that the development of sympathetic neurons and chromaffin cells is impaired in mouse embryos with a conditional deletion of Islet-1 controlled by the wnt1 promotor. Islet-1 is not essential for the initial differentiation of sympathoadrenal cells, as indicated by the correct expression of pan-neuronal and catecholaminergic subtype specific genes in primary sympathetic ganglia of Islet-1 deficient mouse embryos. However, our data indicate that the subsequent survival of sympathetic neuron precursors and their differentiation towards TrkA expressing neurons depends on Islet-1 function. In contrast to spinal sensory neurons, sympathetic neurons of Islet-1 deficient mice did not display ectopic expression of genes normally present in the CNS. In Islet-1 deficient mouse embryos the numbers of chromaffin cells were only mildly reduced, in contrast to that of sympathetic neurons, but the initiation of the adrenaline synthesizing enzyme PNMT was abrogated and the expression level of chromogranin A was diminished. Microarray analysis revealed that developing chromaffin cells of Islet-1 deficient mice displayed normal expression levels of TH, DBH and the transcription factors Phox2B, Mash-1, Hand2, Gata3 and Insm1, but the expression levels of the transcription factors Gata2 and Hand1, and AP-2ß were significantly reduced. Together our data indicate that Islet-1 is not essentially required for the initial differentiation of sympathoadrenal cells, but has an important function for the correct subsequent development of sympathetic neurons and chromaffin cells.
Keywords: Chromaffin cell; Islet-1; Mouse; Sympathetic neuron; Sympathoadrenal cell lineage.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Lineage and stage specific requirement for Dicer1 in sympathetic ganglia and adrenal medulla formation and maintenance.Dev Biol. 2015 Apr 15;400(2):210-23. doi: 10.1016/j.ydbio.2015.01.026. Epub 2015 Feb 4. Dev Biol. 2015. PMID: 25661788
-
Generation of neuroendocrine chromaffin cells from sympathoadrenal progenitors: beyond the glucocorticoid hypothesis.Ann N Y Acad Sci. 2002 Oct;971:554-9. doi: 10.1111/j.1749-6632.2002.tb04526.x. Ann N Y Acad Sci. 2002. PMID: 12438182 Review.
-
Development of chromaffin cells depends on MASH1 function.Development. 2002 Oct;129(20):4729-38. doi: 10.1242/dev.129.20.4729. Development. 2002. PMID: 12361965
-
The Gata3 transcription factor is required for the survival of embryonic and adult sympathetic neurons.J Neurosci. 2010 Aug 11;30(32):10833-43. doi: 10.1523/JNEUROSCI.0175-10.2010. J Neurosci. 2010. PMID: 20702712 Free PMC article.
-
The chromaffin cell and its development.Neurochem Res. 2005 Jun-Jul;30(6-7):921-5. doi: 10.1007/s11064-005-6966-5. Neurochem Res. 2005. PMID: 16187226 Review.
Cited by
-
Temporal requirements for ISL1 in sympathetic neuron proliferation, differentiation, and diversification.Cell Death Dis. 2018 Feb 14;9(2):247. doi: 10.1038/s41419-018-0283-9. Cell Death Dis. 2018. PMID: 29445148 Free PMC article.
-
ISL1 promoted tumorigenesis and EMT via Aurora kinase A-induced activation of PI3K/AKT signaling pathway in neuroblastoma.Cell Death Dis. 2021 Jun 15;12(6):620. doi: 10.1038/s41419-021-03894-3. Cell Death Dis. 2021. PMID: 34131100 Free PMC article.
-
RNA-seq of Isolated Chromaffin Cells Highlights the Role of Sex-Linked and Imprinted Genes in Adrenal Medulla Development.Sci Rep. 2019 Mar 8;9(1):3929. doi: 10.1038/s41598-019-40501-0. Sci Rep. 2019. PMID: 30850723 Free PMC article.
-
ISL1 is necessary for auditory neuron development and contributes toward tonotopic organization.Proc Natl Acad Sci U S A. 2022 Sep 13;119(37):e2207433119. doi: 10.1073/pnas.2207433119. Epub 2022 Sep 8. Proc Natl Acad Sci U S A. 2022. PMID: 36074819 Free PMC article.
-
Lineage-Selective Dependencies in Pediatric Cancers.Cold Spring Harb Perspect Med. 2025 Apr 1;15(4):a041573. doi: 10.1101/cshperspect.a041573. Cold Spring Harb Perspect Med. 2025. PMID: 38806246 Free PMC article. Review.
References
-
- Abellan A, Menuet A, Dehay C, Medina L, Rétaux S. Differential expression of LIM-homeodomain factors in Cajal-Retzius cells of primates, rodents, and birds. Cereb Cortex. 2010;20:1788–1798. - PubMed
-
- Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. - PubMed
-
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. - PubMed
-
- Anderson DJ, Axel R. A bipotential neuroendocrine precursor whose choice of cell fate is determined by NGF and glucocorticoids. Cell. 1986;47:1079–1090. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

