Amyloid-β positron emission tomography imaging probes: a critical review
- PMID: 23648516
- PMCID: PMC3740015
- DOI: 10.3233/JAD-130485
Amyloid-β positron emission tomography imaging probes: a critical review
Abstract
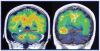
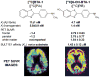
The rapidly rising prevalence and cost of Alzheimer's disease in recent decades has made the imaging of amyloid-β deposits the focus of intense research. Several amyloid imaging probes with purported specificity for amyloid-β plaques are currently at various stages of FDA approval. However, a number of factors appear to preclude these probes from clinical utilization. As the available "amyloid specific" positron emission tomography imaging probes have failed to demonstrate diagnostic value and have shown limited utility for monitoring therapeutic interventions in humans, a debate on their significance has emerged. The aim of this review is to identify and discuss critically the scientific issues contributing to the extensive inconsistencies reported in the literature on their purported in vivo amyloid specificity and potential utilization in patients.
Figures
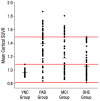
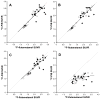
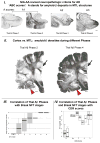
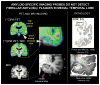
References
-
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. - PubMed
-
- Villemagne VL, Klunk WE, Mathis CA, Rowe CC, Brooks DJ, Hyman BT, Ikonomovic MD, Ishii K, Jack CR, Jagust WJ, Johnson KA, Koeppe RA, Lowe VJ, Masters CL, Montine TJ, Morris JC, Nordberg A, Petersen RC, Reiman EM, Selkoe DJ, Sperling RA, Van Laere K, Weiner MW, Drzezga A. Aβ Imaging: feasible, pertinent, and vital to progress in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2012;39:209–219. - PMC - PubMed
-
- Fleisher AS, Chen K, Liu X, Roontiva A, Thiyyagura P, Ayutyanont N, Joshi AD, Clark CM, Mintun MA, Pontecorvo MJ, Doraiswamy PM, Johnson KA, Skovronsky DM, Reiman EM. Using positron emission tomography and florbetapir F18 to image cortical amyloid in patients with mild cognitive impairment or dementia due to Alzheimer disease. Arch Neurol. 2011;68:1404–1411. - PubMed
-
- Lee H, Zhu X, Castellani RJ, Nunomura A, Perry G, Smith MA. Amyloid-β in Alzheimer disease: The null versus the alternate hypotheses. J Pharm Experimental Therap. 2007;321:823–829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

