JUN regulates early transcriptional responses to axonal injury in retinal ganglion cells
- PMID: 23648575
- PMCID: PMC3700614
- DOI: 10.1016/j.exer.2013.04.021
JUN regulates early transcriptional responses to axonal injury in retinal ganglion cells
Abstract
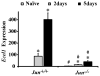
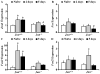
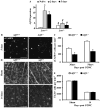
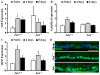
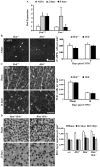
The AP1 family transcription factor JUN is an important molecule in the neuronal response to injury. In retinal ganglion cells (RGCs), JUN is upregulated soon after axonal injury and disrupting JUN activity delays RGC death. JUN is known to participate in the control of many different injury response pathways in neurons, including pathways controlling cell death and axonal regeneration. The role of JUN in regulating genes involved in cell death, ER stress, and regeneration was tested to determine the overall importance of JUN in regulating RGC response to axonal injury. Genes from each of these pathways were transcriptionally controlled following axonal injury and Jun deficiency altered the expression of many of these genes. The differentially expressed genes included, Atf3, Ddit3, Ecel1, Gadd45α, Gal, Hrk, Pten, Socs3, and Sprr1a. Two of these genes, Hrk and Atf3, were tested for importance in RGC death using null alleles of each gene. Disruption of the prodeath Bcl2 family member Hrk did not affect the rate or amount of RGC death after axonal trauma. Deficiency in the ATF/CREB family transcription factor Atf3 did lessen the amount of RGC death after injury, though it did not provide long term protection to RGCs. Since JUN's dimerization partner determines its transcriptional targets, the expression of several candidate AP1 family members were examined. Multiple AP1 family members were induced by axonal injury and had a different expression profile in Jun deficient retinas compared to wildtype retinas (Fosl1, Fosl2 and Jund). Overall, JUN appears to play a multifaceted role in regulating RGC response to axonal injury.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

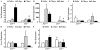
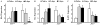
References
-
- Aguilera C, Nakagawa K, Sancho R, Chakraborty A, Hendrich B, Behrens A. c-Jun N-terminal phosphorylation antagonises recruitment of the Mbd3/NuRD repressor complex. Nature. 2011;469:231–235. - PubMed
-
- Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974;13:771–783. - PubMed
-
- Bahr M. Live or let die - retinal ganglion cell death and survival during development and in the lesioned adult CNS. Trends Neurosci. 2000;23:483–490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

