Small airway obstruction in COPD: new insights based on micro-CT imaging and MRI imaging
- PMID: 23648907
- PMCID: PMC3653349
- DOI: 10.1378/chest.12-1766
Small airway obstruction in COPD: new insights based on micro-CT imaging and MRI imaging
Abstract
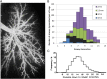
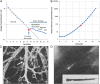
The increase in total cross-sectional area in the distal airways of the human lung enhances the mixing of each tidal breath with end-expiratory gas volume by slowing bulk flow and increasing gas diffusion. However, this transition also favors the deposition of airborne particulates in this region because they diffuse 600 times slower than gases. Furthermore, the persistent deposition of toxic airborne particulates stimulates a chronic inflammatory immune cell infiltration and tissue repair and remodeling process that increases the resistance in airways <2 mm in diameter four to 40-fold in COPD. This increase was originally attributed to lumen narrowing because it increases resistance in proportion to the change in lumen radius raised to the fourth power. In contrast, removal of one-half the number of tubes arranged in parallel is required to double their resistance, and approximately 90% need to be removed to explain the increase in resistance measured in COPD. However, recent reexamination of this problem based on micro-CT imaging indicates that terminal bronchioles are both narrowed and reduced to 10% of the control values in the centrilobular and 25% in the panlobular emphysematous phenotype of very severe (GOLD [Global Initiative for Chronic Obstructive Lung Disease] grade IV) COPD. These new data indicate that both narrowing and reduction in numbers of terminal bronchioles contribute to the rapid decline in FEV₁ that leads to severe airway obstruction in COPD. Moreover, the observation that terminal bronchiolar loss precedes the onset of emphysematous destruction suggests this destruction begins in the very early stages of COPD.
Figures

Similar articles
-
Small-airway obstruction and emphysema in chronic obstructive pulmonary disease.N Engl J Med. 2011 Oct 27;365(17):1567-75. doi: 10.1056/NEJMoa1106955. N Engl J Med. 2011. PMID: 22029978 Free PMC article.
-
Analysis of airway pathology in COPD using a combination of computed tomography, micro-computed tomography and histology.Eur Respir J. 2018 Feb 14;51(2):1701245. doi: 10.1183/13993003.01245-2017. Print 2018 Feb. Eur Respir J. 2018. PMID: 29444912 Free PMC article.
-
What drives the peripheral lung-remodeling process in chronic obstructive pulmonary disease?Proc Am Thorac Soc. 2009 Dec;6(8):668-72. doi: 10.1513/pats.200907-079DP. Proc Am Thorac Soc. 2009. PMID: 20008873 Free PMC article. Review.
-
Dysfunctional lung anatomy and small airways degeneration in COPD.Int J Chron Obstruct Pulmon Dis. 2013;8:7-13. doi: 10.2147/COPD.S28290. Epub 2013 Jan 4. Int J Chron Obstruct Pulmon Dis. 2013. PMID: 23319856 Free PMC article. Review.
-
Small airways disease in mild and moderate chronic obstructive pulmonary disease: a cross-sectional study.Lancet Respir Med. 2018 Aug;6(8):591-602. doi: 10.1016/S2213-2600(18)30196-6. Epub 2018 Jul 4. Lancet Respir Med. 2018. PMID: 30072106
Cited by
-
Consensus Statements on Deployment-Related Respiratory Disease, Inclusive of Constrictive Bronchiolitis: A Modified Delphi Study.Chest. 2023 Mar;163(3):599-609. doi: 10.1016/j.chest.2022.10.031. Epub 2022 Nov 4. Chest. 2023. PMID: 36343686 Free PMC article.
-
Micro-Computed Tomography Comparison of Preterminal Bronchioles in Centrilobular and Panlobular Emphysema.Am J Respir Crit Care Med. 2017 Mar 1;195(5):630-638. doi: 10.1164/rccm.201602-0278OC. Am J Respir Crit Care Med. 2017. PMID: 27611890 Free PMC article.
-
Upregulation of Notch Signaling and Cell-Differentiation Inhibitory Transcription Factors in Stable Chronic Obstructive Pulmonary Disease Patients.Int J Mol Sci. 2024 Mar 14;25(6):3287. doi: 10.3390/ijms25063287. Int J Mol Sci. 2024. PMID: 38542260 Free PMC article.
-
An overview of exacerbations of chronic obstructive pulmonary disease: Can tests of small airways' function guide diagnosis and management?Ann Thorac Med. 2020 Apr-Jun;15(2):54-63. doi: 10.4103/atm.ATM_323_19. Epub 2020 Apr 3. Ann Thorac Med. 2020. PMID: 32489439 Free PMC article. Review.
-
The Contribution of Small Airway Obstruction to the Pathogenesis of Chronic Obstructive Pulmonary Disease.Physiol Rev. 2017 Apr;97(2):529-552. doi: 10.1152/physrev.00025.2015. Physiol Rev. 2017. PMID: 28151425 Free PMC article. Review.
References
-
- Rabe KF, Hurd S, Anzueto A, et al. ; Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532-555. - PubMed
-
- Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645-2653. - PubMed
-
- Kumar V, Abbas A, Fausto N. Tissue renewal and repair: regeneration, healing and fibrosis. In: Robbins & Cotran Pathologic Basis of Disease. 7th ed Elsevier Saunders; 2005:87-118.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

