Tetrahydrobiopterin has a glucose-lowering effect by suppressing hepatic gluconeogenesis in an endothelial nitric oxide synthase-dependent manner in diabetic mice
- PMID: 23649519
- PMCID: PMC3749361
- DOI: 10.2337/db12-1242
Tetrahydrobiopterin has a glucose-lowering effect by suppressing hepatic gluconeogenesis in an endothelial nitric oxide synthase-dependent manner in diabetic mice
Abstract
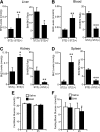
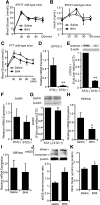
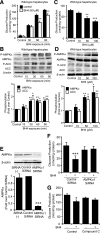
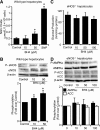
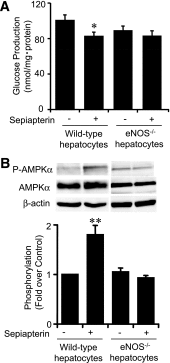
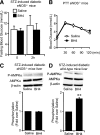
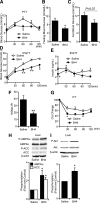
Endothelial nitric oxide synthase (eNOS) dysfunction induces insulin resistance and glucose intolerance. Tetrahydrobiopterin (BH4) is an essential cofactor of eNOS that regulates eNOS activity. In the diabetic state, BH4 is oxidized to 7,8-dihydrobiopterin, which leads to eNOS dysfunction owing to eNOS uncoupling. The current study investigates the effects of BH4 on glucose metabolism and insulin sensitivity in diabetic mice. Single administration of BH4 lowered fasting blood glucose levels in wild-type mice with streptozotocin (STZ)-induced diabetes and alleviated eNOS dysfunction by increasing eNOS dimerization in the liver of these mice. Liver has a critical role in glucose-lowering effects of BH4 through suppression of hepatic gluconeogenesis. BH4 activated AMP kinase (AMPK), and the suppressing effect of BH4 on gluconeogenesis was AMPK-dependent. In addition, the glucose-lowering effect and activation of AMPK by BH4 did not appear in mice with STZ-induced diabetes lacking eNOS. Consecutive administration of BH4 in ob/ob mice ameliorated glucose intolerance and insulin resistance. Taken together, BH4 suppresses hepatic gluconeogenesis in an eNOS-dependent manner, and BH4 has a glucose-lowering effect as well as an insulin-sensitizing effect in diabetic mice. BH4 has potential in the treatment of type 2 diabetes.
Figures
References
-
- Triggle CR, Ding H. A review of endothelial dysfunction in diabetes: a focus on the contribution of a dysfunctional eNOS. J Am Soc Hypertens 2010;4:102–115 - PubMed
-
- Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 2006;113:1888–1904 - PubMed
-
- Duplain H, Burcelin R, Sartori C, et al. . Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 2001;104:342–345 - PubMed
-
- Shankar RR, Wu Y, Shen HQ, et al. . Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes 2000;49:684–687 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

