A common landscape for membrane-active peptides
- PMID: 23649542
- PMCID: PMC3719082
- DOI: 10.1002/pro.2274
A common landscape for membrane-active peptides
Abstract
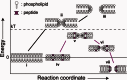
Three families of membrane-active peptides are commonly found in nature and are classified according to their initial apparent activity. Antimicrobial peptides are ancient components of the innate immune system and typically act by disruption of microbial membranes leading to cell death. Amyloid peptides contribute to the pathology of diverse diseases from Alzheimer's to type II diabetes. Preamyloid states of these peptides can act as toxins by binding to and permeabilizing cellular membranes. Cell-penetrating peptides are natural or engineered short sequences that can spontaneously translocate across a membrane. Despite these differences in classification, many similarities in sequence, structure, and activity suggest that peptides from all three classes act through a small, common set of physical principles. Namely, these peptides alter the Brownian properties of phospholipid bilayers, enhancing the sampling of intrinsic fluctuations that include membrane defects. A complete energy landscape for such systems can be described by the innate membrane properties, differential partition, and the associated kinetics of peptides dividing between surface and defect regions of the bilayer. The goal of this review is to argue that the activities of these membrane-active families of peptides simply represent different facets of what is a shared energy landscape.
Copyright © 2013 The Protein Society.
Figures
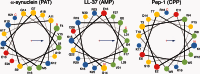

References
-
- Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today. 2012;17:850–860. - PubMed
-
- Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. - PubMed
-
- Butterfield SM, Lashuel HA. Amyloidogenic protein-membrane interactions: mechanistic insight from model systems. Angew Chem Int Ed Engl. 2010;49:5628–5654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

