Heat and radiofrequency plasma glow discharge pretreatment of a titanium alloy promote bone formation and osseointegration
- PMID: 23649564
- PMCID: PMC3786157
- DOI: 10.1002/jcb.24585
Heat and radiofrequency plasma glow discharge pretreatment of a titanium alloy promote bone formation and osseointegration
Abstract
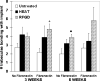
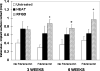
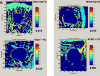
Orthopedic and dental implants manifest increased failure rates when inserted into low density bone. We determined whether chemical pretreatments of a titanium alloy implant material stimulated new bone formation to increase osseointegration in vivo in trabecular bone using a rat model. Titanium alloy rods were untreated or pretreated with heat (600°C) or radiofrequency plasma glow discharge (RFGD). The rods were then coated with the extracellular matrix protein fibronectin (1 nM) or left uncoated and surgically implanted into the rat femoral medullary cavity. Animals were euthanized 3 or 6 weeks later, and femurs were removed for analysis. The number of trabeculae in contact with the implant surface, surface contact between trabeculae and the implant, and the length and area of bone attached to the implant were measured by histomorphometry. Implant shear strength was measured by a pull-out test. Both pretreatments and fibronectin enhanced the number of trabeculae bonding with the implant and trabeculae-to-implant surface contact, with greater effects of fibronectin observed with pretreated compared to untreated implants. RFGD pretreatment modestly increased implant shear strength, which was highly correlated (r(2) = 0.87-0.99) with measures of trabecular bonding for untreated and RFGD-pretreated implants. In contrast, heat pretreatment increased shear strength 3-5-fold for both uncoated and fibronectin-coated implants at 3 and 6 weeks, suggesting a more rapid increase in implant-femur bonding compared to the other groups. In summary, our findings suggest that the heat and RFGD pretreatments can promote the osseointegration of a titanium alloy implant material.
Keywords: BONE MINERALIZATION; CELL DIFFERENTIATION; DENTAL IMPLANT; FIBRONECTIN; OSSEOINTEGRATION; OSTEOBLAST.
Copyright © 2013 Wiley Periodicals, Inc. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures













References
-
- Adell R, Eriksen B, Lekholm U, Brånemark PI, Jemt T. A long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants. 1990;5:347–359. - PubMed
-
- Barber TA, Ho JE, DeRanieri A, Virdi AS, Sumner DR, Healy KE. Peri-implant bone formation and implant integration strength of peptide-modified p(AAM-co-EG/AAC) interpenetrating polymer network-coated titanium implants. J Biomed Mater Res A. 2007;80:306–320. - PubMed
-
- Boskey A, Mendelsohn R. Infrared analysis of bone in health and disease. J Biomed Opt. 2005;10:31–102. - PubMed
-
- Cooper LF. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J Prosth Dent. 2000;84:522–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

