Conditional deletion of neurogenin-3 using Nkx2.1iCre results in a mouse model for the central control of feeding, activity and obesity
- PMID: 23649822
- PMCID: PMC3759333
- DOI: 10.1242/dmm.011916
Conditional deletion of neurogenin-3 using Nkx2.1iCre results in a mouse model for the central control of feeding, activity and obesity
Abstract
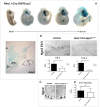
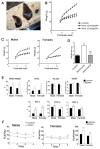
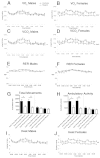
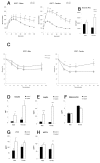
The ventral hypothalamus acts to integrate visceral and systemic information to control energy balance. The basic helix-loop-helix transcription factor neurogenin-3 (Ngn3) is required for pancreatic β-cell development and has been implicated in neuronal development in the hypothalamus. Here, we demonstrate that early embryonic hypothalamic inactivation of Ngn3 (also known as Neurog3) in mice results in rapid post-weaning obesity that is associated with hyperphagia and reduced energy expenditure. This obesity is caused by loss of expression of Pomc in Pomc- and Cart-expressing (Pomc/Cart) neurons in the arcuate nucleus, indicating an incomplete specification of anorexigenic first order neurons. Furthermore, following the onset of obesity, both the arcuate and ventromedial hypothalamic nuclei become insensitive to peripheral leptin treatment. This conditional mouse mutant therefore represents a novel model system for obesity that is associated with hyperphagia and underactivity, and sheds new light upon the roles of Ngn3 in the specification of hypothalamic neurons controlling energy balance.
Figures

References
-
- Chen H., Simar D., Lambert K., Mercier J., Morris M. J. (2008). Maternal and postnatal overnutrition differentially impact appetite regulators and fuel metabolism. Endocrinology 149, 5348–5356 - PubMed
-
- Cowley M. A., Smart J. L., Rubinstein M., Cerdán M. G., Diano S., Horvath T. L., Cone R. D., Low M. J. (2001). Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

