The mTERF protein MOC1 terminates mitochondrial DNA transcription in the unicellular green alga Chlamydomonas reinhardtii
- PMID: 23649833
- PMCID: PMC3711419
- DOI: 10.1093/nar/gkt313
The mTERF protein MOC1 terminates mitochondrial DNA transcription in the unicellular green alga Chlamydomonas reinhardtii
Abstract
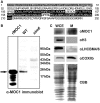
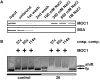
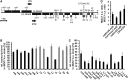
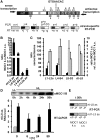
The molecular function of mTERFs (mitochondrial transcription termination factors) has so far only been described for metazoan members of the protein family and in animals they control mitochondrial replication, transcription and translation. Cells of photosynthetic eukaryotes harbour chloroplasts and mitochondria, which are in an intense cross-talk that is vital for photosynthesis. Chlamydomonas reinhardtii is a unicellular green alga widely used as a model organism for photosynthesis research and green biotechnology. Among the six nuclear C. reinhardtii mTERF genes is mTERF-like gene of Chlamydomonas (MOC1), whose inactivation alters mitorespiration and interestingly also light-acclimation processes in the chloroplast that favour the enhanced production of biohydrogen. We show here from in vitro studies that MOC1 binds specifically to a sequence within the mitochondrial rRNA-coding module S3, and that a knockout of MOC1 in the mutant stm6 increases read-through transcription at this site, indicating that MOC1 acts as a transcription terminator in vivo. Whereas the level of certain antisense RNA species is higher in stm6, the amount of unprocessed mitochondrial sense transcripts is strongly reduced, demonstrating that a loss of MOC1 causes perturbed mitochondrial DNA (mtDNA) expression. Overall, we provide evidence for the existence of mitochondrial antisense RNAs in C. reinhardtii and show that mTERF-mediated transcription termination is an evolutionary-conserved mechanism occurring in phototrophic protists and metazoans.
Figures

References
-
- Asin-Cayuela J, Gustafsson CM. Mitochondrial transcription and its regulation in mammalian cells. Trends Biochem. Sci. 2007;32:111–117. - PubMed
-
- Lipinski KA, Kaniak-Golik A, Golik P. Maintenance and expression of the S. cerevisiae mitochondrial genome—from genetics to evolution and systems biology. Biochim. Biophys. Acta. 2010;1797:6–7. - PubMed
-
- Vahrenholz C, Riemen G, Pratje E, Dujon B, Michaelis G. Mitochondrial DNA of Chlamydomonas reinhardtii: the structure of the ends of the linear 15.8-kb genome suggests mechanisms for DNA replication. Curr. Genet. 1993;24:241–247. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

