Presynaptic pH and vesicle fusion in Drosophila larvae neurones
- PMID: 23649934
- PMCID: PMC4282566
- DOI: 10.1002/syn.21678
Presynaptic pH and vesicle fusion in Drosophila larvae neurones
Abstract
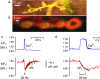
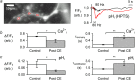
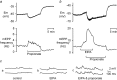
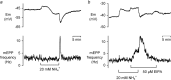
Both intracellular pH (pHi) and synaptic cleft pH change during neuronal activity yet little is known about how these pH shifts might affect synaptic transmission by influencing vesicle fusion. To address this we imaged pH- and Ca(2+) -sensitive fluorescent indicators (HPTS, Oregon green) in boutons at neuromuscular junctions. Electrical stimulation of motor nerves evoked presynaptic Ca(2+) i rises and pHi falls (∼0.1 pH units) followed by recovery of both Ca(2+) i and pHi. The plasma-membrane calcium ATPase (PMCA) inhibitor, 5(6)-carboxyeosin diacetate, slowed both the calcium recovery and the acidification. To investigate a possible calcium-independent role for the pHi shifts in modulating vesicle fusion we recorded post-synaptic miniature end-plate potential (mEPP) and current (mEPC) frequency in Ca(2+) -free solution. Acidification by propionate superfusion, NH(4)(+) withdrawal, or the inhibition of acid extrusion on the Na(+)/H(+) exchanger (NHE) induced a rise in miniature frequency. Furthermore, the inhibition of acid extrusion enhanced the rise induced by propionate addition and NH(4)(+) removal. In the presence of NH(4)(+), 10 out of 23 cells showed, after a delay, one or more rises in miniature frequency. These findings suggest that Ca(2+) -dependent pHi shifts, caused by the PMCA and regulated by NHE, may stimulate vesicle release. Furthermore, in the presence of membrane permeant buffers, exocytosed acid or its equivalents may enhance release through positive feedback. This hitherto neglected pH signalling, and the potential feedback role of vesicular acid, could explain some important neuronal excitability changes associated with altered pH and its buffering.
Keywords: Na+/H+ exchanger; exocytosis; intracellular pH; neuromuscular junction; synaptic transmission.
Copyright © 2013 Wiley Periodicals, Inc.
Figures
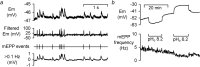

References
-
- Augustine GJ. How does calcium trigger neurotransmitter release? Current Opin Neurobiol. 2001;11:320–326. - PubMed
-
- Balasubramanyam M, Gardner JP. Protein kinase C modulates cytosolic free calcium by stimulating calcium pump activity in Jurkat T cells. Cell Calcium. 1995;18:526–541. - PubMed
-
- Behrendorff N, Floetenmeyer M, Schwiening C, Thorn P. Protons released during pancreatic acinar cell secretion acidify the lumen and contribute to pancreatitis in mice. Gastroenterology. 2010;139:1711–1720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

