Physicochemical and functional comparability between the proposed biosimilar rituximab GP2013 and originator rituximab
- PMID: 23649935
- PMCID: PMC3775154
- DOI: 10.1007/s40259-013-0036-3
Physicochemical and functional comparability between the proposed biosimilar rituximab GP2013 and originator rituximab
Abstract
Background: Regulatory approval for a biosimilar product is provided on the basis of its comparability to an originator product. A thorough physicochemical and functional comparability exercise is a key element in demonstrating biosimilarity. Here we report the characterization of a proposed biosimilar rituximab (GP2013) and originator rituximab.
Objective: To compare GP2013 with originator rituximab using an extensive array of routine analytical and extended characterization methods.
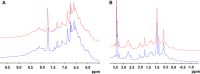
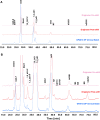
Methods: Primary and higher order protein structures were analyzed using a variety of methods that included high-performance liquid chromatography electrospray ionization mass spectrometry (HPLC-ESI-MS), peptide mapping with UV and MS detection, circular dichroism (CD), Fourier transform infrared (FTIR) spectroscopy, hydrogen deuterium exchange (HDX) MS, 1D (1)H nuclear magnetic resonance (NMR) spectroscopy, X-ray crystallography and differential scanning calorimetry (DSC). Charge and amino acid modifications were assessed using cation exchange chromatography (CEX) and peptide mapping using reversed-phase (RP) HPLC. Boronate affinity chromatography was used to determine the relative amount of glycation. Glycans were identified and quantified after 2-aminobenzamide (2-AB) labeling and separation using normal phase HPLC with fluorescence and MS detection, respectively. Glycan site occupancy was determined using reducing capillary electrophoresis with sodium dodecyl sulfate (CE-SDS). Size heterogeneity was determined using reducing and non-reducing CE-SDS, size exclusion chromatography (SEC) and asymmetric flow field flow fractionation (AF4). Biological characterization included a series of bioassays (in vitro target binding, antibody-dependent cell-mediated cytotoxicity [ADCC], complement-dependent cytotoxicity [CDC] and apoptosis) and surface plasmon resonance (SPR) Fc receptor binding assays.
Results: Intact mass analysis of GP2013 and the heavy and light chains using RP HPLC-ESI-MS revealed the expected molecular mass of rituximab. The amino acid sequence was shown to be identical between GP2013 and the originator rituximab. Further sequence confirmation using RP-HPLC-UV/MS peptide mapping showed non-distinguishable chromatograms for Lys-C digested GP2013 and originator rituximab. The higher order structure of GP2013 was shown to be indistinguishable from originator rituximab using a large panel of redundant and orthogonal methods. GP2013 and originator rituximab were comparable with regard to charge variants, specific amino acid modifications and the glycan pattern. GP2013 was also shown to have similar purity, aggregate and particle levels when compared with the originator. Functionally, and by using a comprehensive set of bioassays and binding assays covering a broad range of rituximab's functional activities, GP2013 could not be distinguished from originator rituximab.
Conclusion: GP2013 was shown to be physicochemically highly similar to originator rituximab at the level of primary and higher order structure, post-translational modifications and size variants. An extensive functional characterization package indicated that GP2013 has the same biological properties as originator rituximab.
Figures




References
-
- European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on similar biological medicinal products containing monoclonal antibodies—non-clinical and clinical issues. London: European Medicines Agency; 2012. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin....
-
- Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Guidance for Industry. Scientific considerations in demonstrating biosimilarity to a reference product. Rockville: Food and Drug Administration; 2012. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

