Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes
- PMID: 23650073
- PMCID: PMC4624280
- DOI: 10.1002/glia.22483
Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes
Abstract
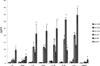
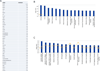
Inflammation is a common component of acute injuries of the central nervous system (CNS) such as ischemia, and degenerative disorders such as Alzheimer's disease. Glial cells play important roles in local CNS inflammation, and an understanding of the roles for microRNAs in glial reactivity in injury and disease settings may therefore lead to the development of novel therapeutic interventions. Here, we show that the miR-181 family is developmentally regulated and present in high amounts in astrocytes compared to neurons. Overexpression of miR-181c in cultured astrocytes results in increased cell death when exposed to lipopolysaccharide (LPS). We show that miR-181 expression is altered by exposure to LPS, a model of inflammation, in both wild-type and transgenic mice lacking both receptors for the inflammatory cytokine TNF-α. Knockdown of miR-181 enhanced LPS-induced production of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β, IL-8) and HMGB1, while overexpression of miR-181 resulted in a significant increase in the expression of the anti-inflammatory cytokine IL-10. To assess the effects of miR-181 on the astrocyte transcriptome, we performed gene array and pathway analysis on astrocytes with reduced levels of miR-181b/c. To examine the pool of potential miR-181 targets, we employed a biotin pull-down of miR-181c and gene array analysis. We validated the mRNAs encoding MeCP2 and X-linked inhibitor of apoptosis as targets of miR-181. These findings suggest that miR-181 plays important roles in the molecular responses of astrocytes in inflammatory settings. Further understanding of the role of miR-181 in inflammatory events and CNS injury could lead to novel approaches for the treatment of CNS disorders with an inflammatory component.
Copyright © 2013 Wiley Periodicals, Inc.
Figures
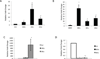
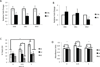


References
-
- Baune BT, Wiede F, Braun A, Golledge J, Arolt V, Koerner H. Cognitive dysfunction in mice deficient for TNF-α and its receptors. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1056–1064. - PubMed
-
- Boldin MP, Baltimore D. MicroRNAs, new effectors and regulators of NF-κB. Immunol Rev. 2012;246:205–220. - PubMed
-
- Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Mattson MP. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

