Can intermittent dosing optimize prolonged linezolid treatment of difficult multidrug-resistant tuberculosis?
- PMID: 23650165
- PMCID: PMC3697330
- DOI: 10.1128/AAC.00388-13
Can intermittent dosing optimize prolonged linezolid treatment of difficult multidrug-resistant tuberculosis?
Abstract
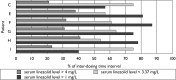
We evaluated treatment with linezolid, dosed at 800 mg once daily for 1 to 4 months as guided by sputum culture status and tolerance and then at 1,200 mg thrice weekly until ≥ 1 year after culture conversion, in addition to individually optimized regimens among 10 consecutive patients with extensively drug-resistant tuberculosis or fluoroquinolone-resistant multidrug-resistant tuberculosis. All achieved stable cure, with anemia corrected and neuropathy stabilized, ameliorated, or avoided after switching to intermittent dosing. Serum linezolid profiles appeared better optimized.
Figures
References
-
- World Health Organization 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. WHO/HTM/TB/2010.3. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf
-
- Sotgiu G, Centis R, D'Ambrosio L, Alffenaar JW, Anger HA, Caminero JA, Castiglia P, De Lorenzo S, Ferrara G, Koh WJ, Schecter GF, Shim TS, Singla R, Skrahina A, Spanevello A, Udwadia ZF, Villar M, Zampogna E, Zellweger JP, Zumla A, Migliori GB. 2012. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: systematic review and meta-analysis. Eur. Respir. J. 40:1430–1442 - PubMed
-
- Bressler AM, Zimmer SM, Gilmore JL, Somani J. 2004. Peripheral neuropathy associated with prolonged use of linezolid. Lancet Infect. Dis. 4:528–531 - PubMed
-
- Koh W-J, Kwon OJ, Gwak H, Chung JW, Cho S-N, Kim WS, Shim TS. 2009. Daily 300 mg dose of linezolid for the treatment of intractable multidrug-resistant and extensively drug-resistant tuberculosis. J. Antimicrob. Chemother. 64:388–391 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources