Cytomegalovirus UL97 kinase catalytic domain mutations that confer multidrug resistance
- PMID: 23650173
- PMCID: PMC3697320
- DOI: 10.1128/AAC.00511-13
Cytomegalovirus UL97 kinase catalytic domain mutations that confer multidrug resistance
Abstract
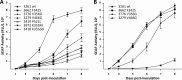
Human cytomegalovirus UL97 kinase mutations that commonly confer ganciclovir resistance cluster in different parts of the gene than those conferring resistance to maribavir, an experimental UL97 kinase inhibitor. The drug resistance, growth, and autophosphorylation phenotypes of several unusual UL97 mutations in the kinase catalytic domain were characterized. Mutations V466G and P521L, described in clinical specimens from ganciclovir-treated subjects, conferred a UL97 kinase knockout phenotype with no autophosphorylation, a severe growth defect, and high-level ganciclovir, cyclopropavir, and maribavir resistance, similar to mutations at the catalytic lysine residue K355. Mutations F342S and V356G, observed after propagation under cyclopropavir in vitro, showed much less growth attenuation and moderate- to high-level resistance to all three drugs while maintaining UL97 autophosphorylation competence and normal cytopathic effect in cell culture, a novel phenotype. F342S is located in the ATP-binding P-loop and is homologous to a c-Abl kinase mutation conferring resistance to imatinib. UL97 mutants with relatively preserved growth fitness and multidrug resistance are of greater concern in antiviral therapy than the severely growth-impaired UL97 knockout mutants. Current diagnostic genotyping assays are unlikely to detect F342S and V356G, and the frequency of their appearance in clinical specimens remains undefined.
Figures

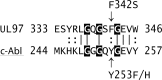
Similar articles
-
Cyclopropavir inhibits the normal function of the human cytomegalovirus UL97 kinase.Antimicrob Agents Chemother. 2011 Oct;55(10):4682-91. doi: 10.1128/AAC.00571-11. Epub 2011 Jul 25. Antimicrob Agents Chemother. 2011. PMID: 21788463 Free PMC article.
-
Ganciclovir and maribavir susceptibility phenotypes of cytomegalovirus UL97 ATP binding region mutations detected by expanded genotypic testing.Antiviral Res. 2021 Sep;193:105139. doi: 10.1016/j.antiviral.2021.105139. Epub 2021 Jul 14. Antiviral Res. 2021. PMID: 34273445 Free PMC article.
-
Cytomegalovirus UL97 kinase mutations that confer maribavir resistance.J Infect Dis. 2007 Jul 1;196(1):91-4. doi: 10.1086/518514. Epub 2007 May 17. J Infect Dis. 2007. PMID: 17538888
-
Cytomegalovirus UL97 mutations in the era of ganciclovir and maribavir.Rev Med Virol. 2008 Jul-Aug;18(4):233-46. doi: 10.1002/rmv.574. Rev Med Virol. 2008. PMID: 18383425 Review.
-
Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir.Rev Med Virol. 2009 Jul;19(4):215-29. doi: 10.1002/rmv.615. Rev Med Virol. 2009. PMID: 19434630 Free PMC article. Review.
Cited by
-
Anti-CMV therapy, what next? A systematic review.Front Microbiol. 2023 Nov 20;14:1321116. doi: 10.3389/fmicb.2023.1321116. eCollection 2023. Front Microbiol. 2023. PMID: 38053548 Free PMC article. Review.
-
Novel UL97 drug resistance mutations identified at baseline in a clinical trial of maribavir for resistant or refractory cytomegalovirus infection.Antiviral Res. 2019 Dec;172:104616. doi: 10.1016/j.antiviral.2019.104616. Epub 2019 Sep 27. Antiviral Res. 2019. PMID: 31568799 Free PMC article. Clinical Trial.
-
Advances in the genotypic diagnosis of cytomegalovirus antiviral drug resistance.Antiviral Res. 2020 Apr;176:104711. doi: 10.1016/j.antiviral.2020.104711. Epub 2020 Jan 12. Antiviral Res. 2020. PMID: 31940472 Free PMC article. Review.
-
Genetic Variants Associated with Drug Resistance of Cytomegalovirus in Hematopoietic Cell Transplantation Recipients.Viruses. 2023 May 30;15(6):1286. doi: 10.3390/v15061286. Viruses. 2023. PMID: 37376586 Free PMC article.
-
Ganciclovir and maribavir cross-resistance revisited: Relative drug susceptibilities of canonical cytomegalovirus mutants.Antiviral Res. 2024 Feb;222:105792. doi: 10.1016/j.antiviral.2023.105792. Epub 2023 Dec 30. Antiviral Res. 2024. PMID: 38163624 Free PMC article.
References
-
- Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Snydman DR, Allen U, Humar A. 2010. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation 89:779–785 - PubMed
-
- Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L, Young JA, Rodriguez T, Maertens J, Schmitt M, Einsele H, Ferrant A, Lipton JH, Villano SA, Chen H, Boeckh M. 2011. Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect. Dis. 11:284–292 - PubMed
-
- Winston DJ, Saliba F, Blumberg E, Abouljoud M, Garcia-Diaz JB, Goss JA, Clough L, Avery R, Limaye AP, Ericzon BG, Navasa M, Troisi RI, Chen H, Villano SA, Uknis ME. 2012. Efficacy and safety of maribavir dosed at 100 mg orally twice daily for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, double-blind, multicenter controlled trial. Am. J. Transplant. 12:3021–3030 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

