Use of antihypotensive therapies in extremely preterm infants
- PMID: 23650301
- PMCID: PMC3666108
- DOI: 10.1542/peds.2012-2779
Use of antihypotensive therapies in extremely preterm infants
Abstract
Objective: To investigate the relationships among blood pressure (BP) values, antihypotensive therapies, and in-hospital outcomes to identify a BP threshold below which antihypotensive therapies may be beneficial.
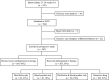
Methods: Prospective observational study of infants 23(0/7) to 26(6/7) weeks' gestational age. Hourly BP values and antihypotensive therapy use in the first 24 hours were recorded. Low BP was investigated by using 15 definitions. Outcomes were examined by using regression analysis controlling for gestational age, the number of low BP values, and illness severity.
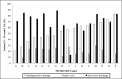
Results: Of 367 infants enrolled, 203 (55%) received at least 1 antihypotensive therapy. Treated infants were more likely to have low BP by any definition (P < .001), but for the 15 definitions of low BP investigated, therapy was not prescribed to 3% to 49% of infants with low BP and, paradoxically, was administered to 28% to 41% of infants without low BP. Treated infants were more likely than untreated infants to develop severe retinopathy of prematurity (15% vs 8%, P = .03) or severe intraventricular hemorrhage (22% vs 11%, P < .01) and less likely to survive (67% vs 78%, P = .02). However, with regression analysis, there were no significant differences between groups in survival or in-hospital morbidity rates.
Conclusions: Factors other than BP contributed to the decision to use antihypotensive therapies. Infant outcomes were not improved with antihypotensive therapy for any of the 15 definitions of low BP investigated.
Trial registration: ClinicalTrials.gov NCT00874393.
Keywords: antihypotensive therapy; blood pressure; extremely preterm infant; hypotension.
Figures

References
-
- Dempsey EM, Barrington KJ. Treating hypotension in the preterm infant: when and with what: a critical and systematic review. J Perinatol. 2007;27(8):469–478 - PubMed
-
- Batton B, Batton D, Riggs T. Blood pressure during the first 7 days in premature infants born at postmenstrual age 23 to 25 weeks. Am J Perinatol. 2007;24(2):107–115 - PubMed
-
- Fanaroff JM, Wilson-Costello DE, Newman NS, Montpetite MM, Fanaroff AA. Treated hypotension is associated with neonatal morbidity and hearing loss in extremely low birth weight infants. Pediatrics. 2006;117(4):1131–1135 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- U10 HD021385/HD/NICHD NIH HHS/United States
- UL1 RR025764/RR/NCRR NIH HHS/United States
- U10 HD053124/HD/NICHD NIH HHS/United States
- U10 HD053119/HD/NICHD NIH HHS/United States
- U10 HD021364/HD/NICHD NIH HHS/United States
- U10 HD027871/HD/NICHD NIH HHS/United States
- UL1 RR025747/RR/NCRR NIH HHS/United States
- UG1 HD053109/HD/NICHD NIH HHS/United States
- UL1 RR025008/RR/NCRR NIH HHS/United States
- U10 HD027856/HD/NICHD NIH HHS/United States
- U10 HD021373/HD/NICHD NIH HHS/United States
- UL1 RR024139/RR/NCRR NIH HHS/United States
- U10 HD027880/HD/NICHD NIH HHS/United States
- K23 HD068497/HD/NICHD NIH HHS/United States
- U10 HD053109/HD/NICHD NIH HHS/United States
- M01 RR008084/RR/NCRR NIH HHS/United States
- UL1 TR001449/TR/NCATS NIH HHS/United States
- U10 HD040689/HD/NICHD NIH HHS/United States
- U10 HD040492/HD/NICHD NIH HHS/United States
- U10 HD027853/HD/NICHD NIH HHS/United States
- U10 HD027904/HD/NICHD NIH HHS/United States
- UL1 TR000041/TR/NCATS NIH HHS/United States
- U10 HD027851/HD/NICHD NIH HHS/United States
- U10 HD034216/HD/NICHD NIH HHS/United States
- U10 HD036790/HD/NICHD NIH HHS/United States
- U10 HD053089/HD/NICHD NIH HHS/United States
- UL1 RR024979/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

