Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling
- PMID: 23650378
- PMCID: PMC3677476
- DOI: 10.1073/pnas.1219997110
Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling
Abstract
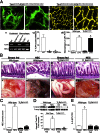
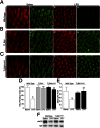
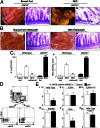
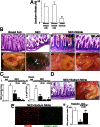
Necrotizing enterocolitis (NEC) is a devastating disease of premature infants characterized by severe intestinal necrosis and for which breast milk represents the most effective protective strategy. Previous studies have revealed a critical role for the lipopolysaccharide receptor toll-like receptor 4 (TLR4) in NEC development through its induction of mucosal injury, yet the reasons for which intestinal ischemia in NEC occurs in the first place remain unknown. We hypothesize that TLR4 signaling within the endothelium plays an essential role in NEC development by regulating perfusion to the small intestine via the vasodilatory molecule endothelial nitric oxide synthase (eNOS). Using a unique mouse system in which we selectively deleted TLR4 from the endothelium, we now show that endothelial TLR4 activation is required for NEC development and that endothelial TLR4 activation impairs intestinal perfusion without effects on other organs and reduces eNOS expression via activation of myeloid differentiation primary response gene 88. NEC severity was significantly increased in eNOS(-/-) mice and decreased upon administration of the phosphodiesterase inhibitor sildenafil, which augments eNOS function. Strikingly, compared with formula, human and mouse breast milk were enriched in sodium nitrate--a precursor for enteral generation of nitrite and nitric oxide--and repletion of formula with sodium nitrate/nitrite restored intestinal perfusion, reversed the deleterious effects of endothelial TLR4 signaling, and reduced NEC severity. These data identify that endothelial TLR4 critically regulates intestinal perfusion leading to NEC and reveal that the protective properties of breast milk involve enhanced intestinal microcirculatory integrity via augmentation of nitrate-nitrite-NO signaling.
Keywords: infant formula; neonatal inflammation; neonatal nutrition; prematurity; sepsis.
Conflict of interest statement
Conflict of interest statement: M.T.G. holds a patent for the use of nitrite salts in cardiovascular diseases and consults with Aires Pharmaceuticals.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000005/TR/NCATS NIH HHS/United States
- P01 HL103455/HL/NHLBI NIH HHS/United States
- P01HL103455/HL/NHLBI NIH HHS/United States
- R01DK08752/DK/NIDDK NIH HHS/United States
- R01 DK083752/DK/NIDDK NIH HHS/United States
- P50 GM053789/GM/NIGMS NIH HHS/United States
- R01GM078238/GM/NIGMS NIH HHS/United States
- R01 GM078238/GM/NIGMS NIH HHS/United States
- R01HL098032/HL/NHLBI NIH HHS/United States
- F30 DK085930/DK/NIDDK NIH HHS/United States
- P50GM053789/GM/NIGMS NIH HHS/United States
- R01 HL096973/HL/NHLBI NIH HHS/United States
- R01HL096973/HL/NHLBI NIH HHS/United States
- K12 HD052892/HD/NICHD NIH HHS/United States
- R01 HL098032/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

