Expression of recombinant human complement C1q allows identification of the C1r/C1s-binding sites
- PMID: 23650384
- PMCID: PMC3666734
- DOI: 10.1073/pnas.1304894110
Expression of recombinant human complement C1q allows identification of the C1r/C1s-binding sites
Abstract
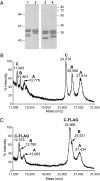
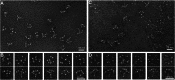
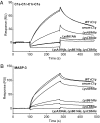
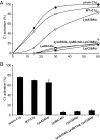
Complement C1q is a hexameric molecule assembled from 18 polypeptide chains of three different types encoded by three genes. This versatile recognition protein senses a wide variety of immune and nonimmune ligands, including pathogens and altered self components, and triggers the classical complement pathway through activation of its associated proteases C1r and C1s. We report a method for expression of recombinant full-length human C1q involving stable transfection of HEK 293-F mammalian cells and fusion of an affinity tag to the C-terminal end of the C chain. The resulting recombinant (r) C1q molecule is similar to serum C1q as judged from biochemical and structural analyses and exhibits the characteristic shape of a bunch of flowers. Analysis of its interaction properties by surface plasmon resonance shows that rC1q retains the ability of serum C1q to associate with the C1s-C1r-C1r-C1s tetramer, to recognize physiological C1q ligands such as IgG and pentraxin 3, and to trigger C1r and C1s activation. Functional analysis of rC1q variants carrying mutations of LysA59, LysB61, and/or LysC58, in the collagen-like stems, demonstrates that LysB61 and LysC58 each play a key role in the interaction with C1s-C1r-C1r-C1s, with LysA59 being involved to a lesser degree. We propose that LysB61 and LysC58 both form salt bridges with outer acidic Ca(2+) ligands of the C1r and C1s CUB (complement C1r/C1s, Uegf, bone morphogenetic protein) domains. The expression method reported here opens the way for deciphering the molecular basis of the unusual binding versatility of C1q by mapping the residues involved in the sensing of its targets and the binding of its receptors.
Keywords: C1 complex; innate immunity; protein engineering.
Conflict of interest statement
Conflict of interest statement: This work is part of a patent application by I.B. and N.M.T.
Figures
References
-
- Cooper NR. The classical complement pathway: Activation and regulation of the first complement component. Adv Immunol. 1985;37:151–216. - PubMed
-
- Gaboriaud C, et al. Structure and activation of the C1 complex of complement: Unraveling the puzzle. Trends Immunol. 2004;25(7):368–373. - PubMed
-
- Bohlson SS, Fraser DA, Tenner AJ. Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol Immunol. 2007;44(1-3):33–43. - PubMed
-
- Ghebrehiwet B, Hosszu KK, Valentino A, Peerschke EI. 2012. The C1q family of proteins: Insights into the emerging non-traditional functions. Front Immunol 3:52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

